Prostate cancer is a heterogeneous disease, the second deadliest malignancy in men and the most commonly diagnosed cancer among men. Traditional plants have been applied to handle various diseases and to develop new drugs. Medicinal plants are potential sources of natural bioactive compounds that include alkaloids, phenolic compounds, terpenes, and steroids.
- prostate cancer
- medicinal plants
- phytotherapy
- secondary metabolites
- plant formulas
1. Introduction
1.1. A Brief Overview on Prostate Cancer
1.2. Prostate Cancer: Main Risk Factors
1.2.1. Non-Modified Risk Factors
|
Risk Group |
Relative Risk of Prostate Cancer |
|---|---|
|
Father and brother had prostate cancer |
9 |
|
≥2 first degree relatives having prostate cancer |
4.39 |
|
Brothers having prostate cancer |
3.14 |
|
First degree relative with prostate cancer at the age of<65 |
2.87 |
|
Second degree relative with prostate cancer |
2.52 |
|
One first degree relative with prostate cancer |
2.48 |
|
Father having prostate cancer |
2.35 |
|
First degree relative with prostate cancer at the age of ≥65 |
1.92 |
1.2.2. Modified Risk Factors
2. Therapeutic Strategies: A Brief Summary
|
Risk Group |
Clinical Stage |
PSA (ng/mL) |
Gleason Score |
Biopsy Criteria |
|---|---|---|---|---|
|
Low |
T1a or T1c |
<10 |
2–6 |
Unilateral or <50% of core involved |
|
Intermediate |
T1b, T1c, or T2a |
<10 |
3 + 4 = 7 |
Bilateral |
|
High |
T1b, T1c, T2b, or T3 |
10–20 |
4 + 3 = 7 |
>50% of core involved or perineural invasion or ductal differentiation |
|
Very high |
T4 |
>20 |
8–10 |
Lymphovascular invasion or neuroendocrine differentiation |
3. Plant Extracts and Plant-Derived Bioactives in Prostate Cancer
3.1. Plant Extracts with Anti-Prostate Cancer Potential
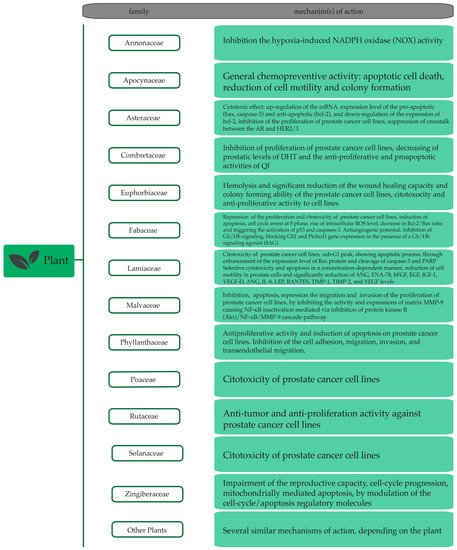
|
Plant Species |
Family |
In Vitro |
In Vivo |
References |
|---|---|---|---|---|
|
Acacia catechu |
Fabaceae |
+ |
- |
[110] |
|
Achillea santolinoides |
Asteraceae |
+ |
- |
[111] |
|
Achillea teretifolia |
Asteraceae |
+ |
- |
[112] |
|
Allium wallichii |
Amaryllidaceae |
+ |
- |
[113] |
|
Aloe perryi |
Xanthorrhoeaceae |
+ |
- |
[114] |
|
Anaxagorea brevipes |
Annonaceae |
+ |
- |
[115] |
|
Angelica gigas |
Apiaceae |
- |
+ |
|
|
Annona muricata |
Annonaceae |
+ |
- |
[118] |
|
Anogeissus latifolia |
Combretaceae |
+ |
- |
[110] |
|
Apocynum venetum |
Apocynaceae |
+ |
- |
[119] |
|
Arachis hypogaea |
Fabaceae |
+ |
- |
[120] |
|
Baliospermum montanum |
Euphorbiaceae |
+ |
+ |
[121] |
|
Berberis libanotica |
Berberidaceae |
+ |
- |
[122] |
|
Byrsonima crassifolia |
Malpighiaceae |
+ |
- |
[123] |
|
Calliandra portoricensis |
Fabaceae |
+ |
- |
[124] |
|
Capsicum chinense |
Solanaceae |
+ |
- |
[123] |
|
Carica papaya |
Caricaceae |
+ |
- |
[125] |
|
Cascabela peruviana |
Apocynaceae |
+ |
- |
[126] |
|
Chenopodium hybridum |
Amaranthaceae |
+ |
- |
[127] |
|
Cnidoscolus chayamansa |
Euphorbiaceae |
+ |
- |
[123] |
|
Cornus mas |
Cornaceae |
+ |
- |
[128] |
|
Costus pulverulentus |
Costaceae |
+ |
- |
[129] |
|
Crataegus Pinnatifida |
Rosaceae |
+ |
- |
[130] |
|
Crocus sativus |
Iridaceae |
+ |
+ |
|
|
Curcuma longa |
Zingiberaceae |
+ |
- |
|
|
Cymbopogon citratus |
Poaceae |
+ |
- |
[135] |
|
Cymbopogon giganteus |
Poaceae |
+ |
- |
[135] |
|
Euphorbia microsciadia |
Euphorbiaceae |
+ |
- |
[111] |
|
Euphorbia szovitsii |
Euphorbiaceae |
+ |
- |
[111] |
|
Eurycoma longifolia |
Simaroubaceae |
+ |
+ |
[136] |
|
Fagara zanthoxyloides |
Rutaceae |
+ |
- |
[137] |
|
Fagopyrum esculentum |
Polygonaceae |
+ |
- |
[138] |
|
Fagopyrum tataricum |
Polygonaceae |
+ |
- |
[138] |
|
Ficus deltoidea var. angustifolia |
Moraceae |
+ |
- |
[139] |
|
Ficus deltoidea var. deltoidea |
Moraceae |
+ |
- |
[139] |
|
Formosa lambsquarters |
Amaranthaceae |
+ |
- |
[138] |
|
Glycine max |
Fabaceae |
+ |
- |
[140] |
|
Glycyrrhiza uralensis |
Fabaceae |
+ |
- |
[141] |
|
Haplophyllum perforatum |
Rutaceae |
+ |
- |
[111] |
|
Helicteres hirsuta |
Malvaceae |
+ |
- |
[142] |
|
Hertia angustifolia |
Asteraceae |
+ |
- |
[111] |
|
Hibiscus sabdariffa |
Malvaceae |
+ |
+ |
[143] |
|
Leucaena leucocephala |
Fabaceae |
+ |
- |
[123] |
|
Lysimachia ciliata |
Primulaceae |
+ |
- |
[144] |
|
Malmea depressa |
Annonaceae |
+ |
- |
[123] |
|
Maytenus royleana |
Celastraceae |
+ |
+ |
[145] |
|
Medicago sativa |
Fabaceae |
+ |
- |
[111] |
|
Melissa officinalis |
Lamiaceae |
+ |
- |
|
|
Mentha arvensis |
Lamiaceae |
+ |
- |
[148] |
|
Mentha spicata |
Lamiaceae |
+ |
- |
[148] |
|
Mentha viridis |
Lamiaceae |
+ |
- |
[148] |
|
Moringa oleifera |
Moringaceae |
+ |
- |
[110] |
|
Nepeta cataria |
Lamiaceae |
+ |
- |
[149] |
|
Nigella sativa |
Ranunculaceae |
+ |
- |
|
|
Oryza sativa |
Poaceae |
+ |
- |
[151] |
|
Paeonia lactiflora |
Paeoniaceae |
+ |
- |
[152]. |
|
Paramignya trimera |
Rutaceae |
+ |
- |
[153] |
|
Phyllanthus amarus |
Phyllanthaceae |
+ |
- |
[154] |
|
Phyllanthus niruri |
Phyllanthaceae |
+ |
- |
[154] |
|
Phyllanthus urinaria |
Phyllanthaceae |
+ |
- |
[154] |
|
Phyllanthus watsonii |
Phyllanthaceae |
+ |
- |
[154] |
|
Plumbago zeylanica |
Plumbaginaceae |
+ |
- |
[155] |
|
Polygonatum sp |
Asparagaceae |
+ |
- |
[156] |
|
Pseudocedrela kotchyi |
Meliaceae |
+ |
- |
[137] |
|
Psidium guajava |
Myrtaceae |
+ |
+ |
|
|
Punica granatum |
Lythraceae |
+ |
+ |
|
|
Quisqualis indica |
Combretaceae |
+ |
+ |
[162] |
|
Remotiflori radix |
Campanulaceae |
+ |
+ |
[163] |
|
Salvia multicaulis Vahl |
Lamiaceae |
+ |
- |
[111] |
|
Salvia trilobal |
Lamiaceae |
+ |
- |
[164] |
|
Sigesbeckia orientalis |
Asteraceae |
+ |
- |
[165] |
|
Sophora alopecuroides |
Fabaceae |
+ |
- |
[111] |
|
Sutherlandia frutescens |
Fabaceae |
+ |
+ |
[166] |
|
Terminalia bellerica |
Combretaceae |
+ |
- |
[110] |
|
Terminalia catappa |
Combretaceae |
+ |
- |
[123] |
|
Urtica dioica |
Urticaceae |
+ |
- |
|
|
Vitis rotundifolia |
Vitaceae |
+ |
- |
[168] |
|
Wedelia chinensis |
Asteraceae |
- |
+ |
|
|
Withania coagulans |
Solanaceae |
- |
+ |
[171] |
|
Xylopia aethiopica |
Annonaceae |
+ |
- |
[172] |
|
Zanthoxyli fructus |
Rutaceae |
+ |
+ |
[173] |
|
Zingiber officinale |
Zingiberaceae |
+ |
+ |
+: Showed in vitro or in vivo antiproliferative effect; -: Not found.
3.2. Plant-Derived Bioactives with Anti-Prostate Cancer Potential
|
Bioactive Compounds |
In Vitro |
In Vivo |
References |
|---|---|---|---|
|
Alkaloids |
|||
|
(−)-Anonaine |
+ |
- |
[182] |
|
(−)-Caaverine |
+ |
- |
[182] |
|
(−)-Nuciferine |
+ |
- |
[182] |
|
6-Hydroxycrinamine |
+ |
- |
[183] |
|
7-Hydroxydehydronuciferine |
+ |
- |
[182] |
|
Capsaicin |
+ |
- |
[184] |
|
Crinamine |
+ |
- |
[183] |
|
Emetine |
+ |
+ |
|
|
Liriodenine |
+ |
- |
[182] |
|
Lycorine |
+ |
+ |
|
|
Matrine |
+ |
- |
[188] |
|
Oxymatrine |
+ |
- |
[188] |
|
Oxysophocarpine |
+ |
- |
[188] |
|
Schisanspheninal A |
+ |
- |
[189] |
|
Sophocarpine |
+ |
- |
[188] |
|
Tetrandrine |
+ |
- |
[190] |
|
Carotenoids |
|||
|
Crocetin |
+ |
- |
[133] |
|
Crocin |
+ |
- |
[132] |
|
Fatty acid |
|||
|
(E)-ethyl 8-methylnon-6-enoate |
+ |
- |
[123] |
|
Phenolic compounds |
|||
|
α-Mangostin |
+ |
+ |
[191]. |
|
γ-Tocopherol |
+ |
- |
[192] |
|
δ-Tocotrienol |
+ |
- |
[192] |
|
(-)-5,7-Difluoroepicatechin-3-O-gallate |
+ |
- |
[193] |
|
(-)-Epicatechin-3-O-gallate |
+ |
- |
[193] |
|
10-Gingerol |
+ |
- |
[175] |
|
6-Gingerol |
+ |
- |
[175] |
|
6-Prenylnaringenin |
+ |
- |
[194] |
|
6-Shogoal |
+ |
- |
[175] |
|
7-o-Galloyl catechin |
+ |
- |
[195] |
|
8-Gingerol |
+ |
- |
[175] |
|
8-Prenylnaringenin |
+ |
- |
[194] |
|
Afzelin |
+ |
- |
[196] |
|
Altholactone |
+ |
- |
[197] |
|
Apigenin |
+ |
[198] |
|
|
Camptothin B |
+ |
- |
[141] |
|
Catechin |
+ |
- |
[195] |
|
Catechin-3-o-gallate |
+ |
- |
[195] |
|
Chlorogenic acid |
+ |
- |
[130] |
|
Chrysin |
+ |
- |
[199] |
|
Cinnamaldehyde |
+ |
- |
[200] |
|
Cornusiin A |
+ |
- |
[141] |
|
Cornusiin H |
+ |
- |
[141] |
|
Curcumin |
+ |
+ |
|
|
Decursin |
+ |
- |
[117] |
|
Decursinol angelate |
+ |
- |
[117] |
|
Dehydrozingerone |
+ |
- |
[205] |
|
Delphinidin |
+ |
+ |
|
|
Ellagic acid |
+ |
+ |
|
|
Eugenol |
+ |
- |
[200] |
|
Fisetin |
+ |
+ |
[210] |
|
Flavokawain A |
+ |
+ |
[211] |
|
Flavopiridol |
+ |
+ |
[212] |
|
Garcinol |
+ |
+ |
|
|
Ginkgetin |
+ |
+ |
[215] |
|
Hesperetin |
+ |
- |
[216] |
|
Hirsutenone |
+ |
- |
[217] |
|
HLBT-100 or HLBT-001 (5,3′-dihydroxy- 6,7,8,4′-tetramethoxyflavanone) |
+ |
- |
[218] |
|
Honokiol |
+ |
- |
[219] |
|
Icarisid II |
+ |
- |
[220] |
|
Isoangustone A |
+ |
- |
|
|
Isovitexin |
+ |
- |
[139] |
|
Juglone |
+ |
- |
[223] |
|
Licoricidin |
+ |
- |
|
|
Magnolol |
+ |
- |
[224] |
|
Mangiferin |
+ |
+ |
|
|
Maysin |
+ |
- |
[227] |
|
Methyl gallate |
+ |
- |
[195] |
|
Osthol |
+ |
- |
|
|
Oxyfadichalcones A |
+ |
- |
[229] |
|
Oxyfadichalcones B |
+ |
- |
[229] |
|
Oxyfadichalcones C |
+ |
- |
[229] |
|
Oxyfadichalcones D |
+ |
- |
[229] |
|
Oxyfadichalcones E |
+ |
- |
[229] |
|
Oxyfadichalcones F |
+ |
- |
[229] |
|
Oxyfadichalcones G |
+ |
- |
[229] |
|
Paeonol |
+ |
+ |
[230] |
|
Peperotetraphin |
+ |
- |
[231] |
|
Physangulatins I |
+ |
- |
[232] |
|
Plumbagin |
+ |
+ |
|
|
Punicalagin |
+ |
- |
[234] |
|
Quercetin |
+ |
+ |
|
|
Resveratrol |
+ |
+ |
|
|
Rutin |
+ |
- |
[241] |
|
Tannic acid |
+ |
- |
[242] |
|
Tricin |
+ |
- |
[243] |
|
Xanthohumol |
+ |
- |
|
|
Protein |
|||
|
Agglutinin |
+ |
+ |
[245] |
|
Diffusa cyclotide 1 |
+ |
- |
[246] |
|
Diffusa cyclotide 2 |
+ |
- |
[246] |
|
Diffusa cyclotide 3 |
+ |
+ |
[246] |
|
Lectin ConBr |
+ |
- |
[247] |
|
Lectin ConM |
+ |
- |
[247] |
|
Lectin DLasiL |
+ |
- |
[247] |
|
Lectin DSclerL |
+ |
- |
[247] |
|
Terpenoids |
|||
|
α-Santalol |
+ |
+ |
[248] |
|
4S,5R,9S,10R-Labdatrien-6,19-olide |
+ |
- |
[249] |
|
(20R)-Dammarane-3β,12β,20,25-tetrol (25-OH-PPD) |
+ |
+ |
[250] |
|
Andrographolide |
+ |
+ |
[251] |
|
Celastrol |
+ |
+ |
[252] |
|
Citral |
+ |
- |
[135] |
|
Diosgenin |
+ |
- |
[253]. |
|
Euphol |
+ |
- |
[254] |
|
Isocuparenal |
+ |
- |
[189] |
|
Jungermannenone A |
+ |
- |
[255] |
|
Jungermannenone B |
+ |
- |
[255] |
|
Muricins M |
+ |
- |
[256] |
|
Muricins N |
+ |
- |
[256] |
|
Nummularic acid |
+ |
- |
[257] |
|
Oenotheralanosterol B |
+ |
- |
[258] |
|
Plectranthoic acid |
+ |
- |
[259] |
|
Sutherlandioside D |
+ |
- |
[166]. |
|
Widdaranal A |
- |
[189] |
|
|
Widdaranal B |
+ |
- |
[189] |
|
Widdarol peroxide |
+ |
- |
[189] |
|
Withaferin A |
+ |
- |
[260] |
-, no effect observed; +, positive effect.
4. Evidence from Clinical Studies
|
Phytochemicals/Formulae |
Bioactive Effect |
Reference |
|---|---|---|
|
Danshen (Salvia miltiorrhiza) |
Protective effects; Improved survival (5–10%) |
[266] |
|
TCM formulae (Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) |
Improved survival |
[267] |
|
Pomegranate juice |
Extension of PSA doubling time, with no adverse effects |
|
|
Pomegranate, green tea, broccoli, turmeric |
Decreased PSA levels |
[271] |
|
Resveratrol |
Decreased the circulating levels of androgen precursors |
[273] |
|
Extension of PSA doubling time, with no adverse effects |
[274] |
|
|
PC-SPEC |
Decreased PSA levels |
[275] |
This entry is adapted from the peer-reviewed paper 10.3390/nu11071483
