Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Stress granules are membrane-less organelles formed through the process of liquid–liquid phase separation (LLPS) under certain stress conditions, such as oxidative stress and heat shock, among others.
- stress granule
- aggrephagy
- neurodegenerative disease
- amyotrophic lateral sclerosis
- phase separation
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by progressive degeneration of the upper and lower motor neurons, resulting in a loss of motor function and eventually death. About 10% of ALS cases are familial (fALS), while about 90% are sporadic (sALS). Identification of ALS-causative genes, including superoxide dismutase 1 (SOD1), transactive response DNA-binding protein 43 (TARDBP-43), fused in sarcoma (FUS), chromosome 9 open reading frame 72 (C9orf72), heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1), valosin-containing protein (VCP), ubiquilin 2 (UBQLN2), sequestosome 1 (SQSTM1/p62), annexin A11 (ANXA11), optineurin (OPTN), and TANK (TRAF-associated NF-κB activator)-binding kinase 1 (TBK1) have advanced the understanding of ALS pathogenesis. ALS gene products are considered resident stress granule (SG) components or SG-associated proteins (Table 1) [1].
Stress granules are membrane-less organelles formed through the process of liquid–liquid phase separation (LLPS) under certain stress conditions, such as oxidative stress and heat shock, among others [1,2]. SGs are transient cellular compartments that undergo dynamic assembly and dissociation. However, chronic stress can lead to persistent stress granules, eventually resulting in the aggregation of disease-related proteins.
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved lysosomal degradative pathway, which is essential in the cellular and organismal levels of homeostasis [3,4,5]. Morphologically, autophagy is initiated by the formation of phagophores in mammalian cells. After nucleation of the phagophore, the membrane expands to generate an autophagosome, which fuses with a lysosome or vacuole, leading to the degradation of the cargo [6,7,8,9]. Clearance of the cytosolic components, such as protein aggregates, is conferred by cargo receptors that specifically recognize the cargo [10,11,12,13,14,15,16,17]. Dysfunction of autophagy is highly associated with various human diseases [18,19,20], such as neurodegenerative diseases.
Protein aggregates derived from interrupted SG dynamics pose a toxic insult, which can be partially mitigated by a selective autophagy pathway called aggrephagy (Figure 1). Aggresomes formed by those insoluble protein aggregates and labeled by ubiquitins are considered to initiate the process of aggrephagy. Aggresomes are transported to a microtubule-organizing center with the help of histone deacetylase 6 (HDAC6), which binds to ubiquitinated cargos [21]. Aggrephagy is controlled by a panel of receptor proteins, such as p62, next to BRCA1 gene 1 (NBR1), toll interacting protein (TOLLIP), OPTN, and Tax1 binding protein 1 (TAXBP1) [22]. Mechanistically, these receptors bridge ubiquitinated protein aggregates with autophagosomal membranes by simultaneously binding to ubiquitin chains and the lipidated LC3-family proteins via ubiquitin-associated (UBA) domains and LC3 interacting region (LIR) motifs, respectively [14,15]. These receptors are able to work both independently and cooperatively. For instance, NBR1 can interact with p62 and promote its phase separation [23]. Autophagy-linked FYVE-domain containing protein (ALFY) interacts with p62 and binds to several autophagy-related proteins, playing a role in the formation of autophagic membranes. The fusion between aggresomes and lysosomes involves proteins including Rab7, marking the final degradation of protein aggregates [21]. It should be noted that the mutations of two of these aggrephagy receptors, p62 and OPTN, are implicated in ALS.
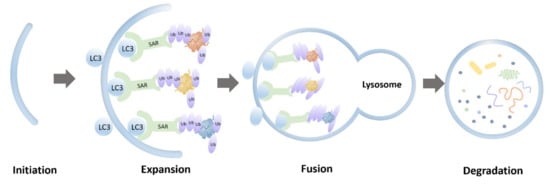
Figure 1. Schematic representation of aggrephagy.
Loss of SG homeostasis and defective aggrephagy are common pathological features of neurogenerative diseases [1,23,24,25,26,27,28]. VCP is encoded by an ALS causal gene and is a critical regulator mediating autophagic degradation of abnormal stress granules [29]. In this review, we will discuss the intersection of aggrephagy and stress granules in the pathogenesis of ALS.
2. Superoxide Dismutase 1 (SOD1)
Superoxide dismutase 1 gene encoding Cu/Zn superoxide dismutase was the first identified ALS-related gene [30]. The enzyme protects cells by detoxifying superoxide radicals O2−. SOD1 gene mutations account for approximately 20% of fALS. Although no consensus linking SOD1 mutations to toxicity has been reached [27], it is generally accepted that ubiquitinated cytoplasmic inclusions formed by ALS-causing SOD1 mutants contribute to toxicity in ALS [27]. Most ALS-associated mutations significantly impact the immature states of SOD1, destabilizing the metal-free and disulfide-reduced polypeptide, which leads to unfolding at physiological temperatures [31]. Moreover, mutations that change the hydrophobicity of SOD1 or cause cellular Ca2+ dysregulation promote the aggregation tendency of SOD1 mutants in ALS [32,33]. In addition, T cell-restricted intracellular antigen 1 (TIA-1) positive SGs can alter the dynamics of stress granules [34].
SOD1 is not a resident protein of SGs. However, mutant SOD1 interacts with TIA-1, one of the core components of stress granules associated with ALS. Mutant SOD1 increases the number of TIA-1 positive SGs. The abnormal interaction between mutant SOD1 and TIA-1 alters the dynamic of stress granules [34]. In addition, mutant SOD1 binds to GTPase-activating protein-(SH3 domain)-binding protein 1 (G3BP1), another protein marker of SGs, in an RNA-independent manner interfering with the dynamics of G3BP1-positive SGs [35]. Therefore, these findings suggest that aberrant interactions between SOD1, TIA-1, and G3BP1 might dysregulate SG.
Mutant SOD1 aggregates can be recognized by p62 and targeted for autophagic degradation [36,37]. Furthermore, mutant SOD1 aggregates may sequester OPTN, resulting in a reduced mitophagy flux, accounting for neurodegeneration [38]. However, whether the perturbation of SGs dynamics by SOD1 mutants impacts protein aggregation tendency remains unclear. Further studies are needed to confirm the exact role of aggrephagy in SOD1-associated ALS and the specific aggrephagy receptors involved [39].
3. Transactive Response DNA-Binding Protein 43 (TDP-43)
Transactive response DNA-binding protein 43 belongs to the heterogeneous ribonucleoprotein family. TDP-43 plays a critical role in diverse cellular processes, such as regulating RNA splicing, pre-microRNA processing, messenger RNA transport, and stress granule formation [40]. Hyper-phosphorylated, ubiquitinated, and cleaved TDP-43 aggregation has been identified as a pathological protein in disease-affected central nervous system regions [41]. Furthermore, TDP-43 has been detected as abnormal cytoplasmic aggregates in neurons and glia of more than 90% of ALS and 45% of frontotemporal dementia (FTD) cases [42].
TDP-43 can aggregate and propagate in a seed-dependent, self-templating, prion-like manner in vitro and in vivo [43]. Under chronic cell stress, TDP-43 is recruited to the cytoplasmic SGs, which evolve to form insoluble pathological aggregates [44,45]. TDP-43 also interacts with the four other ALS causal gene products, HNRNPA1, HNRNPA2B1, matrin 3 (MATR3), and UBQLN2 [46,47,48,49], which are resident proteins in SGs. The identification of TIA-1 as an ALS causal gene further reinforces the fact that TDP-43 in ALS is formed via altered LLPS [50]. These observations suggest that many ALS causal genes may converge on the TDP-43 pathway associated with pathologies.
Several studies have confirmed that autophagy plays a role in clearing TDP-43 aggregates. Significant colocalization between selective autophagy receptor p62 with TPD-43 aggregates was observed in ALS/FTD, indicating that the autophagy pathway could prevent the accumulation of TDP-43 aggregates [51]. In addition, VCP and OPTN appear to colocalize with TDP-43 inclusions in the spinal motor neurons of ALS patients [52]. Upregulation of autophagy leads to reduced TDP-43 proteinopathy in the nervous system of ALS/FTD transgenic mice models, which further validates the role of autophagy in mitigating toxicity of TDP-43 mutants [53,54]. Conversely, TDP-43 also plays a role in the regulation of autophagy by binding to ATG7 mRNA via RNA recognition motif 1(RRM1). Down-regulation of TDP-43 decreases ATG7 mRNA levels, which abolishes autophagosome expansion [55]. Furthermore, the loss of TDP-43 impairs the fusion of autophagosomes with lysosomes through decreasing dynactin 1, a component of the dynein-dynactin complex involved in lysosome transportation. The impaired fusion finally leads to the accumulation of immature autophagic vesicles blocking the autophagy-lysosome pathway [56].
4. Fused in Sarcoma (FUS)
FUS was first discovered in 1993 as a fusion oncogene in human liposarcoma located on chromosome 16 [57,58]. It contains 15 exons encoding a 526-amino acid protein. Moreover, it contains an N-terminal Gln-Gly-Ser-Tyr (QGSY)-rich domain, an RNA-recognition motif, three Arg-Gly-Gly repeat domains (RGG1-3), a zinc-finger motif and a C-terminal nuclear localization signal (NLS) [59]. In 2009, pathological inclusion bodies containing mutant FUS protein were recognized in fALS6 cases [60,61]. Approximately 2/3 of FUS mutations are located on exons 12–15, which encode zinc-finger motif, RGG2 and RGG3 domains, and the NLS. Other mutations are located on exons 3–6, encoding QGSY-rich and RGG1 domains. The C-terminal mutations are twice as likely to occur in fALS than in sALS, while mutations within exons 3–6 are more common in sALS. C-terminal ALS mutations are pathological, as they disrupt NLS [62,63]. They cause defective nuclear import of FUS and cytoplasmic mislocalization. Cytoplasmic FUS mislocalization leads to nuclear loss of function and triggers motor neuron death through a toxic gain of function [64].
Arginine residues in RGG motifs are required for phase separation of FUS. Loss of FUS arginine methylation promotes phase separation and SG association of FUS [65]. Prion-like domains of FUS are located on the QGSY-rich and C-terminal RGG2 domain, contributing to FUS phase separation and aggregation. ALS-associated FUS mutants can bind and sequester wild type (WT) FUS into cytoplasmic SGs [66], accelerating aberrant liquid to solid phase transition of stress granules [67]. The nuclear import receptor (NIR), also known as Transportin-1, recognizes the NLS domain; therefore, it chaperons FUS from the cytoplasm to the nucleus. NIRs can reverse aberrant phase separation and aggregation of proteins with prion-like domains, including FUS and TDP-43, to mitigate neurodegeneration in vivo [65,68].
R521C and P525L are two common FUS mutations associated with ALS. FUS-R521C causes DNA damage and RNA splicing defects [69]. It colocalizes with stress granules, significantly increasing SG assembly and persistence [70]. FUS-R521C-positive SGs were colocalized to LC3-positive autophagosomes accumulating in autophagy-deficient neurons, suggesting that autophagy is involved in the clearance of FUS mutants [71]. P525L FUS mutation causes early-onset of ALS [72]. P525L-positive SGs are more intense and larger than the WT. The PI3K/AKT/mTOR pathway inhibition increases autophagy by reducing FUS recruitment into SGs and reduces abnormal SGs linked to P525L FUS [73]. Accumulation of ubiquitinated proteins and autophagy receptor p62 was detected in neuronal cells with ALS-associated FUS mutation due to impaired autophagy [74]. However, overexpression of Rab1 rescued these defects, suggesting that Rab1 has a protective role in ALS [75].
This entry is adapted from the peer-reviewed paper 10.3390/cells10092247
This entry is offline, you can click here to edit this entry!
