Allelopathy is an ecological phenomenon in which organisms interfere with each other. As a management strategy in agricultural systems, allelopathy can be mainly used to control weeds, resist pests, and disease and improve the interaction of soil nutrition and microorganisms. Volatile organic compounds (VOCs) are allelochemicals volatilized from plants and have been widely demonstrated to have different ecological functions.
- allelochemicals
- VOCs properties
- VOCs action
- VOCs detection
- green agriculture
1. Introduction
The concept of “allelopathy” was first proposed by Austrian scientist Hans Molisch in 1937 and mainly referred to the chemical relationship of plant interaction. Allelopathy is an ecological phenomenon and plays an important role in the ecological adaptation of plants [1][2]. The allelopathic effects have both positive and negative effects. Various studies have reported the advantages of allelopathic effects in agricultural systems, such as weed control [3][4][5][6], inhibition of pests [7][8][9][10], disease [11][12], improvement of soil nutrition [13][14], and microbial interactions [15][16]. Ultimately, allelopathy of most plants has effect on plant growth [10][17][18]. Plants can synthesize various secondary metabolites during growth and development. Plant Volatile organic compounds (VOCs) vary by species, and they are related to the abundance of neighboring plant species and plant species composition [19][20]. These secondary metabolites can be beneficial or harmful to other organisms when stored or released into the environment, such as secondary metabolites stored in plants that can prevent animal feeding and microbial infestation, while volatiles released into the air can attract insect pollinators [21]. Plants communicate with organisms in the environment through VOCs, thereby achieving a wide range of ecological functions, such as affecting their growth, development, defense, reproduction, and life cycle [22]. In 1984, allelopathy was defined as “any direct or indirect harmful effect by one plant (including microorganisms) on another through production of chemical compounds released into the environment” by Rice [23]. These products of secondary metabolism, called allelochemicals, can be found in any organ of the plant (leaves, stems, flowers, seeds, fruits, and/or roots) and can be released from the producing plant by different routes: volatilization, foliar leaching, root exudations, and decomposition of plant residue ( Figure 1 ).
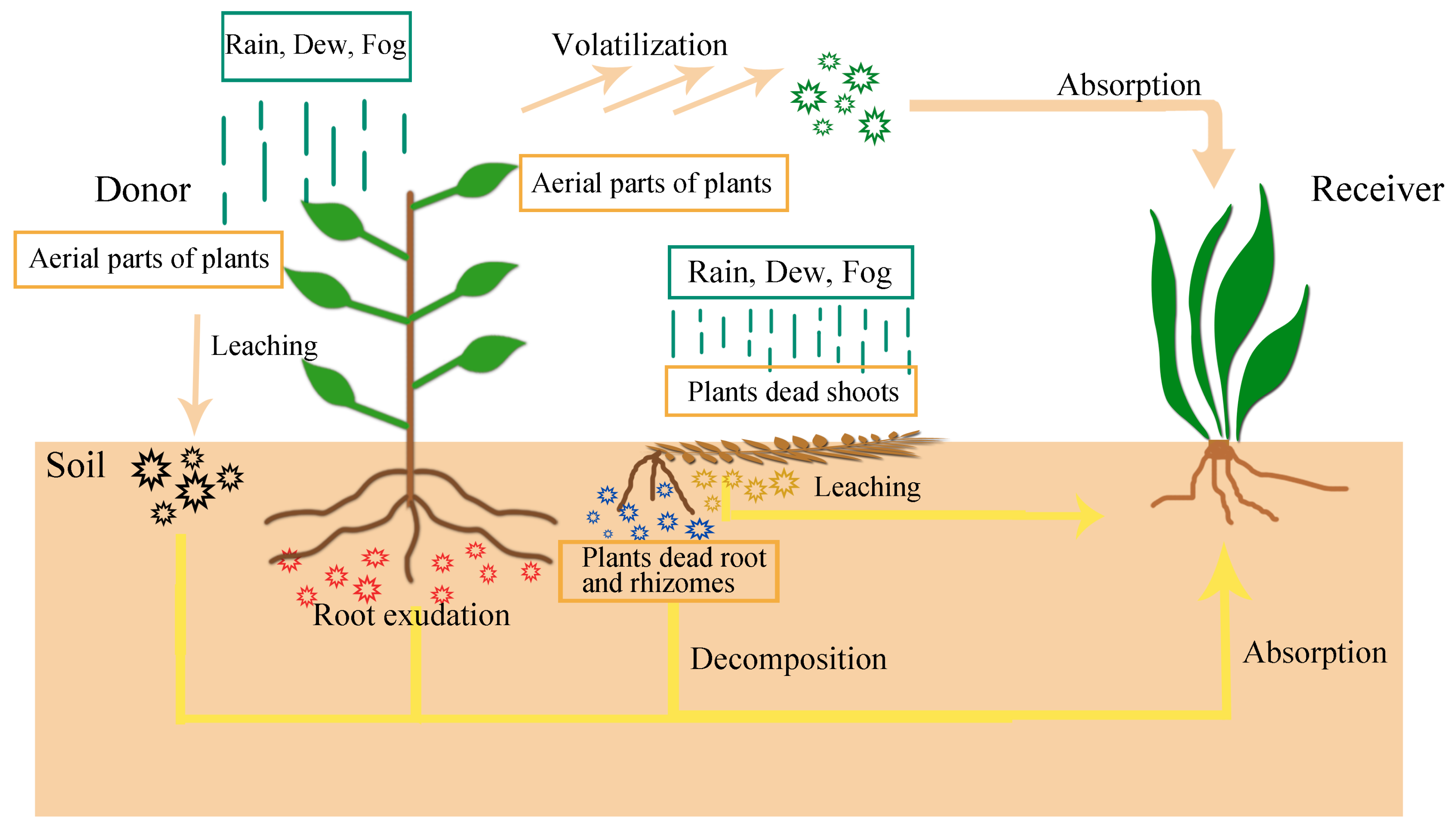
Figure 1. The allelopathy pathways of plants.
VOCs are secondary metabolites volatilized by plants and ubiquitous allelochemicals of plants [24]. Shikimate/phenylalanine, the mevalonic acid (MVA), the methylerythritol phosphate (MEP), and lipoxygenase (LOX) pathways are the four main synthesis pathways of VOCs, and plants can synthesize and release various VOCs including terpenoids, phenylpropanoids/benzenoids, and fatty acid derivatives [25]. The VOCs released by these plants often have different ecological functions, such as chemical communication, kin recognition, attracting or repelling insects, and many other effects [21][26][27][28][29]. Although the researches of plants VOCs are mainly aboveground some chemical signal, more and more studies show that VOCs also play an integral part in belowground plant–plant interactions [30]. In fact, the phenomenon that plants release allelochemicals through the volatile pathway has been noticed for a long time. One of the first empirical studies of allelopathy involving VOCs was researched by Molisch, who found that VOCs released by apples and pears could inhibit potato germination [31]. VOCs have been widely demonstrated to defend primarily against herbivorous insects [32][33], microbes, and pathogens [34][35][36], thereby reducing extreme environmental stress [37][38] and promoting nutritional acquisition [11]. Muller et al. [39] researched the volatiles of annual grassland species in Salvia leucophylla Greene and Artemisia californica communities, and this revealed that volatile allelochemicals had the interspecific allelopathic effects on the woody herbaceous plants, which would negatively affected the recipient plant species [40][41] and changed soil microorganisms [42][43]. Besides, in addition to VOCs released from plant shoots, root volatiles may also have allelopathic effects on neighboring plants; for example, VOCs from big sagebrush ( Artemisia tidentata Nutt.) root inhibited seed germination of wild tobacco ( Nicotiana attenuata ) [44]. Most allelochemicals produced by plant roots are considered as “root exudates” [45], but the few allelochemicals released by volatilization of roots are called VOCs, which play an important ecological role in the soil ecosystem and have not been studied thoroughly [46][47].
Allelopathy has received high attention and become one of the central scientific problems in ecology [48]. Allelopathy is forming an independent scientific system, and we are conducting in-depth and extensive research from both theoretical and practical aspects. VOCs released by plants are one of the main ways to achieve allelopathic effects. It is a more economical, environmentally friendly, and effective measure to use the allelopathic effect of plant natural products to develop the agricultural production [49]. Several excellent reviews have summarized the relevant research on potential applications of VOCs [20][22][49], but the studies on allelopathy of plants VOCs have not been systematically reviewed and reported. VOCs are a kind of natural and environmentally friendly chemical substances that volatilize from plants and are used as natural herbicides and fungicides to protect neighboring plants from stress and increase crop yields [49]. We think VOCs have a much broader range of the potential applications. So, in this context, study on the allelopathy of VOCs is particularly important to the future development of green agriculture. The review mainly focuses on the recent studies of allelopathy of plants VOCs, regarding resisting diseases and preventing pests of plant, impacting on competition (inhibiting weed hazards), breaking dormancy, regulating plant growth, affecting reactive oxygen species (ROS) content and enzyme activity, modulating plant respiration and photosynthesis, and their role as a signal conducting substance. We present the evidence from the references to illustrate these roles to deepen the understanding of allelopathy of plants VOCs.
2. Method of VOCs Collection and Identification
2.1. Collection of Plants VOCs
2.2. Identification of Plants VOCs
3. Conclusions
Herein summarizes the allelopathy of VOCs of plants including growth, competition, dormancy, resistance of pests and diseases, respiration, photosynthesis, ROS content, enzyme activity, and signaling. It also summarizes the main methods of collection and identification of VOCs. The study of allelopathy is quite a complicated work, because it involves a variety of disciplines such as chemistry, ecology, biology, microbiology, and so on. Scientists in these fields need to work together to conduct research. The study of allelopathy on plants VOCs is still a new field. Most of the researches still focus on the expression of the allelopathic phenomenon, but the depth and breadth of them are far from enough, such as the lack of research on allelopathy mechanisms of plants, the relationship between chemical recognition and communication mechanisms and allelopathy mechanisms, and so on. In recent years, we have seen more and more reports on VOCs. VOC transmission, emission, and accumulation are also hot topics in research, which deserve more research attention. There are still many issues that need to be further explored. Plant VOCs deserve more research attention.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae7090278
References
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506.
- Pan, L.; Li, X.-Z.; Yan, Z.-Q.; Guo, H.-R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure-activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277.
- Boydston, R.A.; Morra, M.J.; Borek, V.; Clayton, L.; Vaughn, S.F. Onion and weed response to mustard (Sinapis alba) seed meal. Weed Sci. 2011, 59, 546–552.
- Awan, F.K.; Rasheed, M.; Ashraf, M.; Khurshid, M.Y. Efficacy of brassica sorghum and sunflower aqueous extracts to control wheat weeds under rainfed conditions of pothwar. Pakistan J. Anim. Plant Sci. 2012, 22, 715–721.
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015, 63, 723–747.
- Tavella, L.B.; Lima e Silva, P.S.; Monteiro, A.L.; de Oliveira, V.R.; de Oliveira Fernandes, P.L. Gliricidia sepium intercropping for weed management in immature corn ear production. Rev. Cienc. Agron. 2017, 48, 650–656.
- Avato, P.; D’Addabbo, T.; Leonetti, P.; Argentieri, M.P. Nematicidal potential of brassicaceae. Phytochem. Rev. 2013, 12, 791–802.
- Liu, T.; Cheng, Z.; Meng, H.; Ahmad, I.; Zhao, H. Growth, yield and quality of spring tomato and physicochemical properties of medium in a tomato/garlic intercropping system under plastic tunnel organic medium cultivation. Sci. Hortic. 2014, 170, 159–168.
- Glinwood, R.; Ninkovic, V.; Pettersson, J. Chemical interaction between undamaged plants—Effects on herbivores and natural enemies. Phytochemistry 2011, 72, 1683–1689.
- Singh, A.; Weisser, W.W.; Hanna, R.; Houmgny, R.; Zytynska, S.E. Reduce pests, enhance production: Benefits of intercropping at high densities for okra farmers in Cameroon. Pest Manag. Sci. 2017, 73, 2017–2027.
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83.
- Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M. Effect of preceding and intercropping crops on suppression of lentil damping-off and root rot disease in New Valley—Egypt. Crop Prot. 2012, 32, 41–46.
- Ma, Y.-h.; Fu, S.-l.; Zhang, X.-p.; Zhao, K.; Chen, H.Y.H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178.
- Ndungu-Magiroi, K.W.; Wortmann, C.S.; Kibunja, C.; Senkoro, C.; Mwangi, T.J.K.; Wamae, D.; Kifuko-Koech, M.; Msakyi, J. Maize-bean intercrop response to nutrient application relative to maize sole crop response. Nutr. Cycl. Agroecosyst. 2017, 109, 17–27.
- Bressan, M.; Roncato, M.-A.; Bellvert, F.; Comte, G.; Haichar, F.e.Z.; Achouak, W.; Berge, O. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. Isme J. 2009, 3, 1243–1257.
- Zhao, M.; Jones, C.M.; Meijer, J.; Lundquist, P.-O.; Fransson, P.; Carlsson, G.; Hallin, S. Intercropping affects genetic potential for inorganic nitrogen cycling by root-associated microorganisms in Medicago sativa and Dactylis glomerata. Appl. Soil Ecol. 2017, 119, 260–266.
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of allelopathy in crop production. Int. J. Agric. Biol. 2013, 15, 1367–1378.
- Alemayehu, A.; Tamado, T.; Nigussie, D.; Yigzaw, D.; Kinde, T.; Wortmann, C.S. Maize-common bean intercropping to optimize maize-based crop production. J. Agric. Sci. 2017, 155, 1124–1136.
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 11050.
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 58.
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144.
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907.
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984.
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefevre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388.
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32.
- Kong, C.; Hu, F.; Xu, X.; Zhang, M.; Liang, W. Volatile allelochemicals in the Ageratum conyzoides intercropped citrus orchard and their effects on mites Amblyseius newsami and Panonychus citri. J. Chem. Ecol. 2005, 31, 2193–2203.
- McNickle, G.G.; St Clair, C.C.; Cahill, J.F., Jr. Focusing the metaphor: Plant root foraging behaviour. Trends Ecol. Evol. 2009, 24, 419–426.
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the “cry for help”. Trends Plant Sci. 2010, 15, 167–175.
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6.
- Gfeller, V.; Huber, M.; Foerster, C.; Huang, W.; Koellner, T.G.; Erb, M. Root volatiles in plant-plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963.
- Molish, H. Der Einfluss Einer Pflanze auf die Andere-Allelopathie; Gustav Fischer Verlag: Jena, Germany, 1937.
- Simms, E.L.; Rausher, M.D. Costs and benefits of plant resistance to herbivory. Am. Nat. 1987, 130, 570–581.
- Kim, J.; Felton, G.W. Priming of antiherbivore defensive responses in plants. Insect Sci. 2013, 20, 273–285.
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280.
- Ameye, M.; Audenaert, K.; De Zutter, N.; Steppe, K.; Van Meulebroek, L.; Vanhaecke, L.; De Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against fusarium graminearum but boosts deoxynivalenol production. Plant Physiol. 2015, 167, 1671–1684.
- Siri-Udom, S.; Suwannarach, N.; Lumyong, S. Applications of volatile compounds acquired from Muscodor heveae against white root rot disease in rubber trees (Hevea brasiliensis Mull. Arg.) and relevant allelopathy effects. Fungal Biol. 2017, 121, 573–581.
- Lerdau, M.; Gray, D. Ecology and evolution of light-dependent and light-independent phytogenic volatile organic carbon. New Phytol. 2003, 157, 199–211.
- Cofer, T.M.; Engelberth, M.; Engelberth, J. Green leaf volatiles protect maize (Zea mays) seedlings against damage from cold stress. Plant Cell Environ. 2018, 41, 1673–1682.
- Muller, C.H.; Muller, W.H.; Haines, B.L. Volatile growth Inhibitors produced by aromatic shrubs. Science 1964, 143, 471–473.
- Muller, C.H. Inhibitory terpenes volatilized from salvia shrubs. Bull. Torrey Bot. Club 1965, 92, 38–45.
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624.
- Norton, J.M.; Harman, G.E. Responses of soil microorganisms to volatile exudates from germinating pea seeds. Can. J. Bot. 1985, 63, 1040–1045.
- Won Yun, K.; Kil, B.S.; Han, D.M. Phytotoxic and antimicrobial activity of volatile constituents of Artemisia princeps var. orientalis. J. Chem. Ecol. 1993, 19, 2757–2766.
- Jassbi, A.R.; Zamanizadehnajari, S.; Baldwin, I.T. Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J. Chem. Ecol. 2010, 36, 1398–1407.
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407.
- Lin, C.; Owen, S.M.; Peñuelas, J. Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol. Biochem. 2007, 39, 951–960.
- Delory, B.M.; Delaplace, P.; Fauconnier, M.-L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26.
- Fitter, A. Making allelopathy respectable. Science 2003, 301, 1337–1338.
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to Improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10.
- Brooks, G.T. Comprehensive insect physiology, biochemistry and pharmacology: Edited by G. A. Kerkut and L. I. Gilbert. Pergamon Press, Oxford. 1985. 13 Volumes. 8200 pp approx. £1700.00/$2750.00. ISBN 0 08 026850 1. Insect Biochem. 1985, 15, i–xiv.
- Kimparis, A.; Siatis, N.; Daferera, D.; Tarantilis, P.; Pappas, C.; Polissiou, M. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60.
- Lee, S.N.; Kim, N.S.; Lee, D.S. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2003, 377, 749–756.
- Mutarutwa, D.; Navarini, L.; Lonzarich, V.; Compagnone, D.; Pittia, P. GC-MS aroma characterization of vegetable matrices: Focus on 3-alkyl-2-methoxypyrazines. J. Mass Spectrom. 2018, 53, 871–881.
- Sgorbini, B.; Cagliero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C.; Cordero, C. Strategies for accurate quantitation of volatiles from foods and plant-origin materials: A challenging task. J. Agric. Food Chem. 2019, 67, 1619–1630.
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. NMR-based metabolomics at work in phytochemistry. Phytochem. Rev. 2007, 6, 3–14.
- Marshall, T.L.; Chaffin, C.T.; Makepeace, V.D.; Hoffman, R.M.; Hammaker, R.M.; Fateley, W.G.; Saarinen, P.; Kauppinen, J. Investigation of the effects of resolution on the performance of classical least-squares (CLS) spectral interpretation programs when applied to volatile organic compounds (VOCs) of interest in remote sensing using open-air long-path Fourier transform infrared (FT-IR) spectrometry. J. Mol. Struct. 1994, 324, 19–28.
- Stierlin, É.; Nicolè, F.; Fernandez, X.; Michel, T. Development of a headspace solid-phase microextraction gas chromatography-mass spectrometry method to study volatile organic compounds (VOCs) emitted by lavender roots. Chem. Biodivers. 2019, 16, e1900280.
- Danner, H.; Samudrala, D.; Cristescu, S.M.; Van Dam, N.M. Tracing hidden herbivores: Time-resolved non-invasive analysis of belowground volatiles by proton-transfer-reaction mass spectrometry (PTR-MS). J. Chem. Ecol. 2012, 38, 785–794.
- Capozzi, V.; Lonzarich, V.; Khomenko, I.; Cappellin, L.; Navarini, L.; Biasioli, F. Unveiling the molecular basis of mascarpone cheese aroma: VOCs analysis by SPME-GC/MS and PTR-ToF-MS. Molecules 2020, 25, 1242.
- Lebanov, L.; Ghiasvand, A.; Paull, B. Data handling and data analysis in metabolomic studies of essential oils using GC-MS. J. Chromatogr. A 2021, 1640, 461896.
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 1–13.
