Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
JWH133, a selective, full functional agonist of the CB2 receptor, has been extensively studied for its potent anti-inflammatory, antiviral, and immunomodulatory properties. JWH133 modulates numerous signaling pathways and inhibits inflammatory mediators, including cytokines, chemokines, adhesion molecules, prostanoids, and eicosanoids.
- cannabinoids
- CB2 receptors
- COVID-19
- immunomodulators
- inflammation
- JWH133
- SARS-CoV-2
1. Introduction
Coronavirus disease-2019 (COVID-19), a pandemic and public health emergency caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a deadly disease that is affecting millions of people all over the world because of the non-availability of specific drugs or vaccines [1]. Currently, numerous efforts are underway to discover and develop preventive and therapeutic agents for SARS-CoV-2 infections [2]. Despite availability of the vaccines for COVID-19, identifying candidate drugs which could be effective for therapeutic management of COVID-19 is crucial. The majority of the drugs used in COVID-19 have been repurposed based on antiviral, antibiotic, anti-inflammatory, or immunomodulatory activities [3]. Considering the emergence of COVID-19-related mortality, effective medications are needed to improve patient prognosis and to stem the spread of the virus [3]. Among the numerous therapeutic avenues to be explored, the endocannabinoid system (ECS), which physiologically regulates innate and adaptive immunity, inflammation, pain, and oxidative stress [4] represents an important strategy for therapeutic targeting of hyperimmune-inflammatory responses during COVID-19.
The ECS typically consists of two receptors, cannabinoid receptor type 1 (CB1R) and 2 (CB2R), their endogenous ligands (endocannabinoids) and metabolic enzymes, as well as nonclassical targets of cannabinoids (e.g., transient receptor potential (TRP) channels and peroxisome proliferator-activated receptors) that are major players in the immune system and control a wide variety of diseases involving immune-inflammatory states [5]. The ECS is one of the newest drug targets receiving attention and has an excellent reputation due to the emergence of many successful drugs in the clinic in the past few years [6,7,8]. In the ECS, the CB2R is a G-protein-coupled receptor (GPCR) that, upon activation, regulates immune responses and inflammatory pathways; therefore, CB2R agonists have received enormous interest for possible therapeutic applications owing to their beneficial immunomodulatory, anti-inflammatory, and antioxidant roles, with the absence of psychotropic effects attributable to CB1R activation [9,10].
To date, numerous cannabinoid ligands have been classified as classical, non-classical, aminoalkylindoles, and eicosanoids that have been synthesized. Among the numerous CB2R ligands, JWH133, which was first synthesized by Huffman et al. (2010), has received enormous attention in experimental studies investigating CB2R-dependent pharmacological mechanisms and therapeutic potential [11]. Since its synthesis, it has been shown to be one of the most studied CB2R full functional agonist that exhibits high affinity and approximately 200-fold more selectivity towards CB2R than CB1R. This emerging ligand shows a wide range of therapeutic effects, including cardioprotective, hepatoprotective, neuroprotective, nephroprotective, anticonvulsive, antipsychotic, anticancer, anti-oxidant, anti-inflammatory, immunomodulatory, and antiviral, mediating selective activation of CB2R mimicking as full agonist.
Since the emergence of COVID-19, several drugs, including remdesivir, lopinavir, ritonavir, interferon-β, ribavirin, chloroquine/hydroxychloroquine, azithromycin, tocilizumab, and ivermectin, have appeared as promising therapeutics for COVID-19 [12]. From a pharmacological perspective, these drugs have the potential to either block the virus from entering host cells or prevent viral replication, and attenuate hyperimmune and hyperinflammatory states to prevent the disease progression and complications [3]. The utilization of these drugs in COVID-19 is mostly empirical, based on clinical experience of their therapeutic benefits in the management of previous SARS, Middle East respiratory syndrome, and Ebola virus epidemics.
2. Molecular Docking of JWH133 for its Activity on Mpro
Molecular docking is a powerful technique used to check the binding orientation of ligand into the active site of the target protein. The crystal structure of SARS-CoV-2 main protease (SARS-CoV-2 Mpro was retrieved from Protein Data Bank (PDB—available at http://www.rcsb.org) using the PDB code: 6LU7 [24], (Berman et al., 2015). Dock Prep tool of UCSF Chimera program was used to prepare receptor molecule [25]. During preparation, binding ligand, hetatoms, and the solvents were removed while the hydrogen atoms were added to the structure. The structure of JWH133 was searched and retrieved from PubChem database (CID: 6918505) [26]. The ligand structure was prepared in chimera by adding hydrogen atoms and charges.
The ligand binding residues in the structure of SARS-CoV-2 Mpro was designated to dock JWH133 using the Autodock Vina chimera plugin [27]. The best-docked ligand pose was selected for further analysis. Energy minimization of the docked complex was performed in Chimera using the energy minimization program [25]. A protein-ligand complex was processed and optimized in the free maestro program to refine molecular interactions [28] (Schrödinger, 2018). The molecular 2D interaction image was also generated using the ligand-receptor interaction module of the maestro package (Schrödinger, 2018). The non-covalent interactions were calculated at cutoff radius of 2.50 Å.
Docking of JWH133 into the active site of SARS-CoV-2 Mpro generated several binding poses. The best binding pose with docking energy −6.0 Kcal/mol was selected for molar interaction analysis. Molecular interaction analysis results revealed that JWH133 formed hydrophobic contact with Cys44, Met49, Pro52, Tyr54, Phe140, Leu141, and Met 165 of the target protein (Figure 1). His41, Asn142, Ser144, His163, His164, His172, and Gln189 were involved in polar contacts with target protein (Figure 1). These residues are the key residues, which play an important role in ligand binding. In a recent study, the importance of similar binding pattern of doxycycline, minocycline, lopinavir, oseltamivir, and ritonavir with SARS-CoV-2 Mpro have been highlighted [23,29].
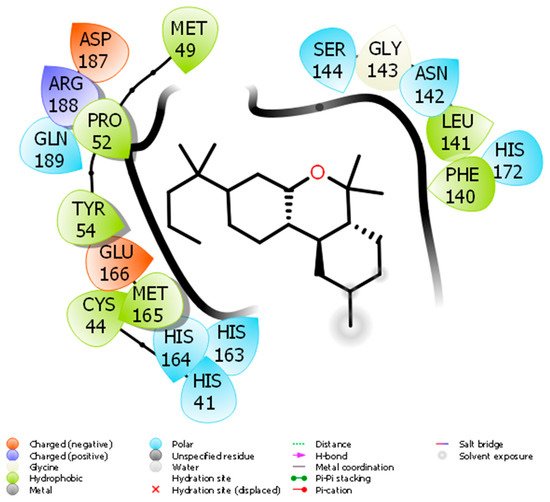
Figure 1. The molecular interaction between SARS-CoV-2 Mpro and JWH133.
Based on the role of CB2R in immune-inflammatory mechanisms, the antiviral and agonist properties of JWH133 on CB2R, we hypothesized that JWH133 may be a potentially novel candidate to limit the severity and progression of COVID-19 by modulating infection, immunity, and inflammation. A scheme of the effect of JWH133 mediating CB2 receptor activation on the infection, inflammation, and immunity in context of SARS-CoV-2 is presented in Figure 2.
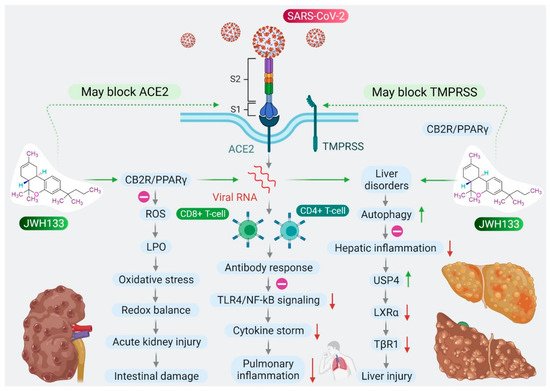
Figure 2. Proposed scheme of potential and mechanisms of JWH133 on immunity, infection, and inflammation against SARS-CoV-2.
3. CB2 Receptors Mediated Anti-Inflammatory Activity of JWH133
The clinical manifestations of SARS-CoV-2 infections range from mild to severe, with widespread participation of the lungs, beginning from pneumonia to acute respiratory distress syndrome (ARDS), as well as acute injury to the liver, heart, intestine, coagulopathy, thrombosis, and neurological manifestations that may lead to sepsis and multi-organ failure with poor prognosis [30,31]. Widespread alveolar damage, along with progressive lung dysfunction, leads to respiratory failure that may cause fatalities [32]. Fatalities are higher in elderly people with cardiometabolic diseases, cancer, patients who are immunocompromised, or with comorbidities of diabetes or cardiometabolic diseases [33]. COVID-19 also causes interstitial lymphopenia, lymphocyte infiltration, and T cell hyperactivation in the lungs and blood [30,31].
CB2Rs are largely expressed in macrophages and participate in the inflammatory process mainly by regulating proinflammatory factors, including cytokines, chemokines, adhesion molecules, and the polarization of macrophages, a key regulator of the M1/M2 pathway of inflammation [34,35]. Activation of CB2R produces anti-inflammatory action by inhibiting leukocyte recruitment, reducing the synthesis and release of proinflammatory cytokines, such as interleukin (IL)-6, IL-18, monocyte chemoattractant protein 1, and reactive oxygen species (ROS) [7]. CB2R primarily couples with Gi/o proteins upon activation, resulting in inhibition of adenylyl cyclase agonism, further activating the 5′ AMP-activated protein kinase (AMPK) pathways that result in reduced anabolic reactions, which, in turn, promote oxidative phosphorylation and exert anti-inflammatory effects [7,36]. Several studies have demonstrated the anti-inflammatory activities of JWH133 in inflammatory models, including lipopolysaccharide (LPS)-induced macrophages, monocytes, and eosinophils by inhibiting proinflammatory cytokines, inflammatory enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, and production of nitric oxide (NO) and prostaglandin E2 [37,38,39].
Patients with COVID-19 mainly present with acute respiratory distress causing acute lung injuries characterized by neutrophil infiltration, vasculitis, and secretion of proinflammatory cytokines, particularly a massive increase in IL-6, which is related to the severity of the disease pathology, poor prognosis, and death [40,41]. Elevated IL-6 levels have also been demonstrated to contribute to acute lung injury (ALI) in murine models [42], similar to those observed in patients with ARDS and COVID-19; thus, inhibition of IL-6 appears to mitigate ALI [42,43]. A few of the potent inhibitors of IL-6 are tocilizumab and sarilumab; these drugs have gained attention in the inhibition of the cytokine storm in COVID-19, but possess numerous adverse effects, such as liver damage, thrombocytopenia, leukopenia, serious infections, gastrointestinal perforations, hypertension, skin reactions, and anaphylaxis [44]. Macrophages present in the human lung express CB2R, which, upon activation, significantly inhibits LPS-induced production of vascular endothelial growth factor-A and C, angiopoietins, and IL-6 secretion [45]. In addition to IL-6, the NOD-like receptor protein 3 (NLRP3) inflammasome is a mediator of the cytokine storm, and, thereby, clinical and pathological manifestations of patients infected with COVID-19 [46]. Recently, JWH133 has been found to exert protective effects in experimental models of ALI by activating CB2R [37,38]. JWH133 significantly inhibits proinflammatory cytokines, including IL-6, and improves levels of antioxidants, mediating the inhibition of inflammasomes [39], the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway [37] and the mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinase (JNK) and nuclear factor-kappa B (NF-κB) pathways [38].
Some patients that have recovered from COVID-19 are reported to have progressive post-infection consequences with persistent lung dysfunction and fibrosis, a life-threatening disease [47]. Pulmonary fibrosis begins with microinjury, resulting in inflammation and over-activation of repair mechanisms following activation of fibroblasts. CB2R present in fibroblasts plays a role in fibrosis, and many studies have demonstrated that activating CB2R exerts anti-inflammatory and antifibrotic effects [48,49,50]. Notably, JWH133, via activation of CB2R, prevents lung fibrosis and reduces fibroblast proliferation, along with suppression of autoantibodies [48]. By activating CB2R, JWH133 also inhibits hyperemia, hyperplasia of type II pneumocytes, interstitial fibrosis and salvaged lungs, reduced fibrotic markers, collagen deposition, decreased levels of the profibrotic cytokine transforming growth factor (TGF)-β1, and mitigated activation of the TGF-β1/mothers against decapentaplegic homolog 2 pathway [49,50].
Additionally, JWH133, mediating CB2R-dependent anti-inflammatory action mitigates neurogenic pulmonary edema developed following subarachnoid hemorrhage, as evidenced by lung permeability, leukocyte trafficking, and preserved tight junctions [51]. Based on the therapeutic and preventive effects of JWH133 in experimental models of ALI, drug-induced lung injuries, inflammation, and fibrosis, as well as airway hyper-responsiveness and cough centers, it is conceivable to speculate that JWH133 may have the potential to curb ALI in COVID-19. It may also limit late-onset pulmonary fibrosis in recovered patients or may be useful in patients with compromised pulmonary function. However, further proof of concept studies is needed for conclusive evidence.
4. CB2 Receptors Mediated Immunomodulatory Activity of JWH133
CB2R is significantly expressed in immunoregulatory cells, including macrophages, B and T cells, and upon activation leads to the subsequent inhibition of cyclic adenosine monophosphate production [109]. CB2R regulates the immune system by controlling immune cell activation through the modulation of T helper cells [110], attenuation of proinflammatory cytokines [111], and NF-κB-mediated apoptosis [112] and found useful in immune-related diseases [113]. CB2R activation has also been shown to mediate immunosuppressive activities of mesenchymal stem cells in immunocompromised conditions [114].
A recent study has demonstrated that JWH133, in combination with dexamethasone, is effective in immune thrombocytopenia purpura (ITP), an autoimmune disease characterized by antibodies against platelets [114]. The combination is effective in mesenchymal stem cells, multipotent cells that have significant roles in immunomodulation and suppress proliferation and activation of both T- and B-lymphocytes, ameliorate apoptotic cell death via B-cell lymphoma 2 signaling, and reinstate the immunomodulatory properties of mesenchymal stem cells derived from patients with ITP [114]. Recently, dexamethasone has been reported to be effective in patients with COVID-19. Thus, JWH133 may reduce the dose of dexamethasone and its adverse effects, along with maintaining its therapeutic effects due to the synergistic combination of dexamethasone and JWH133 [114].
JWH133 prevents the secretion of IL-12p40 and enhances secretion of IL-10 in LPS- or Theiler’s virus-activated macrophages, mediating activation of the CB2R-dependent ERK1/2 MAPK pathway [115]. IL-10 and IL-12 both regulate priming of Th1 or Th2 cells in immune responses. IL-12 plays a significant role in innate and adaptive immunity, and differentiates the immune system towards a Th-1 protective response against viral infections. IL-10 plays a role in maintaining the balance of appropriate macrophage responses to LPS by curbing the synthesis and release of IL-12. CB2R activation in cells belonging to macrophage lineages inhibits the induction of a Th-1 immune response, affecting the required immunity to counter a pathogen or inflammatory state [115].
5. CB2 Receptors Mediated Effects of JWH133 on Acute Lung Injury and Airway Activity
ALI in experimental models is akin to acute respiratory distress in COVID-19. ALI is caused by infections, pneumonia, sepsis, acid aspiration, toxic inhalation, and xenobiotics, which are the major causes of a cytokine storm. CB2R stimulation plays a significant role in protecting the lungs in numerous models of ALI, including cecal ligation puncture-induced septic lung injury [116], I/R-induced lung injury and LPS-induced lung injury [37], paraquat-induced ALI [38], and LPS-induced ALI [117]. JWH133, by activating CB2R, shows potent anti-inflammatory effects in LPS-induced ALI mice by reducing leukocyte migration, vascular permeability, and reducing levels of cytokines, chemokines, and adhesion molecules in the lungs and blood, along with salvaging the lungs [117].
JWH133 has been shown to protect against ALI by inhibiting proinflammatory cytokines, MAPKs, and NF-κB activation via activating CB2R [38], as shown in Figure 3. It also reduces neutrophil infiltration and edema, improving histology of the lungs along with PaO2 in arterial blood [38]. JWH133 has also been found to protect against I/R-induced ALI by reducing levels of cytokines, lipid peroxidation, neutrophil infiltration, lung edema, and improving anti-oxidant and lung histology, along with the PaO2/FiO2 ratio, mediated by the CB2R-dependent PI3K/Akt pathway [37], as shown in Figure 3. Furthermore, JWH133 exerts an antitussive effect in chronic cough by inhibiting activation of sensory nerves in guinea pig and vagus nerves in humans, and suppresses the cough reflex mediated by CB2R activation [118]. In addition to antitussive activity, JWH133 also mitigates bronchoconstriction via inhibition of pre-junctional neurotransmission, neurogenic airway inflammation, and hyper-reactivity [118,119], as shown in Figure 3.
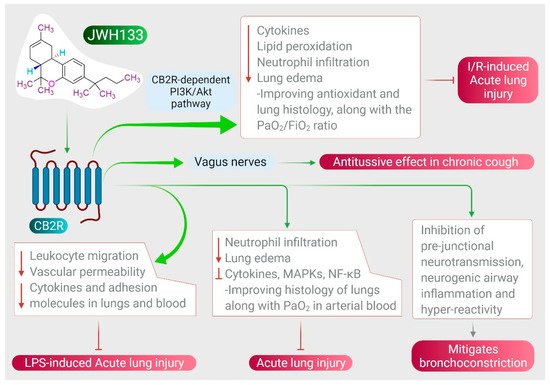
Figure 3. Effect of JWH133 on acute lung injury and airway activity.
Furthermore, at the doses at which cannabinoids produce bronchodilation, JWH133 does not elicit respiratory depression at the central level. CB2R expressed on eosinophils plays a role in lung inflammation mediated by the generation of NO and prostaglandin-E2 [120]. CB2R is involved in antigen processing, immune cell differentiation, and macrophage migration, which have all been shown to play a role in airway immunomodulation [121,122].
6. CB2 Receptors Mediated Anti-Inflammatory and Antiviral Activity of JWH133
CB receptors genetically ablated in mice display an enhanced inflammatory response to influenza infection [123,124]. CB2 gene (CNR2) polymorphisms also play a role in the immunopathogenesis associated with severe necroinflammation in patients with respiratory syncytial virus (RSV) [125], chronic hepatitis C (HCV) [126], childhood ITP [127], celiac disease [127], and necroinflammation in patients with human immunodeficiency virus (HIV)/HCV co-infection [128].
CB2R activation appears to be a novel therapeutic strategy for immunomodulation to improve RSV-induced lung pathology by inhibiting immunoregulatory cells [125]. Recently, JWH133, by CB2R activation, has been shown to exert anti-inflammatory effects by enhancing the production of IL-10, reducing bronchoalveolar influx, inhibiting the release of interferon-γ and macrophage inflammatory protein-1α, and reducing numbers of neutrophils and monocytes in RSV-induced mice. Further, the inhibitory effect of JWH133 on recruitment of neutrophils at the site of inflammation via activation of p38 is additional indication of its anti-inflammatory effects [129].
CB2R has also been shown to be involved in HIV-associated neuropathogenesis by enhancing migration and altering the expression and compartmentation of the β-chemokine receptor CCR-3, as well as releasing inflammatory factors, including the virus-specified trans-activating protein Tat, which further elicits chemokines, cytokines, and a chemotactic response from microglia [130]. Numerous studies have shown that activation of CB2R exerts pleiotropic effects by ameliorating neuroinflammation via inhibiting replication of HIV-1, reducing microglia migration towards HIV-1 Tat, rescuing neurons and endothelial cells, and suppressing viral infection, as well as associated inflammatory responses [131,132,133,134]. CB2R ligands have been shown to suppress replication of HIV-1, rather than interfering with viral entry in microglia [131]. Activation of CB2R significantly suppresses the expression of HIV-1 p24 in microglia and CD4+ T cells in patients infected with HIV-1 [132]. JWH133 also shows significant inhibition of primary CD4+ T cells in HIV-1 infection by inhibiting reorganization and impairing productive infection of C-X-C chemokine receptor type 4-tropic virus [133].
7. CB2 Receptors Mediated Protective Effects of JWH133 in Organ Injuries and Sepsis
Uncontrolled infection and increased inflammatory mediators might cause a systemic inflammatory response and sepsis. CB2R-selective cannabinoids exert potent immunomodulatory and anti-inflammatory effects in the brain, pancreas, intestine, liver, heart, and kidney [34,35]. Activation of CB2R attenuates inflammatory states and oxidative stress in the liver [75,76,77], lungs [50], heart [39], kidney [87], intestine [93], brain [135], and in sepsis [78] by inhibiting inflammatory cell recruitment, proinflammatory cytokines, and increasing levels of anti-inflammatory cytokines.
In a polymicrobial sepsis model in rats [78], JWH133 shows protective effects on brain, lung, liver and, heart, mediated by CB2R activation [78]. JWH133 decreases proinflammatory cytokines and increases the anti-inflammatory cytokine IL-10 [78]. Sepsis is associated with neuronal damage and cognitive impairment, with the participation of proinflammatory cytokines and oxidative/nitrosative stress [78]. Deregulated immunity and an imbalance between the proinflammatory and anti-inflammatory systems results in multi-organ dysfunction and failure, and consequently may cause death. Acute central nervous system (CNS) injury perturbs the homeostasis of the CNS and immune system and enhances patient susceptibility to infections [136]. JWH133 shows neuroprotective effects in LPS-induced neuroinflammation and endotoxemia by mitigating levels of proinflammatory cytokines, adhesion molecules (vascular cell adhesion protein 1 and E-selectin), and oxidative/nitrosative stress [135]. Based on the role of JWH133 in ameliorating sepsis, JWH133 appears to be a potent candidate for limiting COVID-19 progression and post-infection sequelae, including its impact on the multi-organ system.
Furthermore, JWH133 also shows ROS or free radical scavenging and Fe+2 chelating activity against free radicals in numerous in vitro assays, including 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, 2,2-diphenyl-1-picryl-hydrazyl-hydrate, ferric reducing antioxidant power, and oxygen radical absorbance capacity, with chelating and reducing power [137,138], promoting mitochondrial biogenesis [139] and improving endogenous antioxidants in vivo in many tissues. JWH133 inhibits oxidative stress, which initiates and contributes to numerous pathways, including inflammasome activation, nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1), TLR4/high mobility group box 1, MAPK, and sirtuin/PPAR gamma coactivator 1-α (PGC1-α) pathways, leading to the release of inflammatory mediators and cytokines that sustain inflammation, and involving metabolic reprogramming of innate immune cells [77,84,99]. Taken together, JWH133 has been shown to modulate the majority of the signaling pathways that contribute to redox immune-inflammatory signaling those results in organoprotective effects. In addition to the lungs, COVID-19 affects almost all organ systems, including the heart, brain, liver, kidney, intestine, and coagulation system. Thus, the organoprotective effects demonstrated in the in vivo experimental models are encouraging for speculation of the therapeutic benefits of JWH133.
This entry is adapted from the peer-reviewed paper 10.3390/immuno1030020
This entry is offline, you can click here to edit this entry!
