Gelatin is a biopolymer with interesting properties that have greatly attracted the attention of many biomedical researchers, such as low antigenicity, good biodegradability, and biocompatibility in the physiological environment. The gelatin-based materials offer excellent characteristics of wound dressings. The fast degradation time and highly hydrophilic surface make gelatin inappropriate as a base material for the development of wound dressings.
- wound care
- wound dressings
- polymers
- gelatin
- hydrogels
- nanofibers
- sponges
1. Introduction
Wound care is a concern globally with various challenges including the increasing prevalence of type II diabetes, obesity, an aging population, and the need for cost-effective wound dressings [1][2]. The wounds are generally classified based on their healing process as acute or chronic wounds. Acute wounds are lesions that heal within the expected timeframe of approximately 2–3 months depending on the depth and size of the injury in the skin [3]. Chronic wounds fail to heal through the ordinary wound healing process over a prolonged period. Examples of chronic wounds include diabetic wounds, ulcer wounds, burn wounds, etc. [4][5]. All types of wounds require good clinical care to prevent delayed wound healing processes that may be caused by microbial infections and other negative factors. More than 300 types of wound dressing products are available in the market. However, one wound dressing is not appropriate for the treatment of all wound types [6]. In the United States of America (USA), a yearly cost of 20 billion dollars is spent on the wound care of chronic injuries [7]. The global market cost of chronic wound care was 10.12 billion dollars in 2019, and it is projected that the cost will increase to 16.36 billion dollars in 2027 [8]. These statistics demonstrate the negative socio-economic impacts of wound care globally, indicating an urgent need to develop affordable wound dressings for effective wound care.
The properties of an ideal wound dressing that make it suitable to provide a proper environment for the healing process include durability, flexibility, permeability to water vapor, adherence to the tissue, and good mechanical properties [9]. Furthermore, the dressing materials should hydrate/dehydrate the wound, maintain a moist environment, protect the wound from infections, and prevent maceration [10]. Polymer-based wound dressing materials can provide the aforementioned properties. Polymers that can be used for the fabrication of dressings are mainly classified as biopolymers and synthetic polymers [11]. Examples of biopolymers include gelatin, cellulose, chitin, alginate, hyaluronic acid, chitosan, dextran, elastin, fibrin, etc. ( Figure 1 ) [12]. The wound dressings that are formulated from these polymers usually suffer from poor mechanical properties. The combination of biopolymers with synthetic polymers is a promising design strategy to overcome the poor mechanical properties of biopolymer-based wound dressings.
Gelatin is a biopolymer with interesting properties that have greatly attracted the attention of many biomedical researchers, such as low antigenicity, good biodegradability, and biocompatibility in the physiological environment [13]. The gelatin-based materials offer excellent characteristics of wound dressings. The fast degradation time and highly hydrophilic surface make gelatin inappropriate as a base material for the development of wound dressings. Thus, gelatin is combined with other polymers, especially synthetic polymers [14]. This review will discuss the outcomes of gelatin-based hybrid dressings for wound care.
2. Properties of Gelatin in Wound Dressing Applications
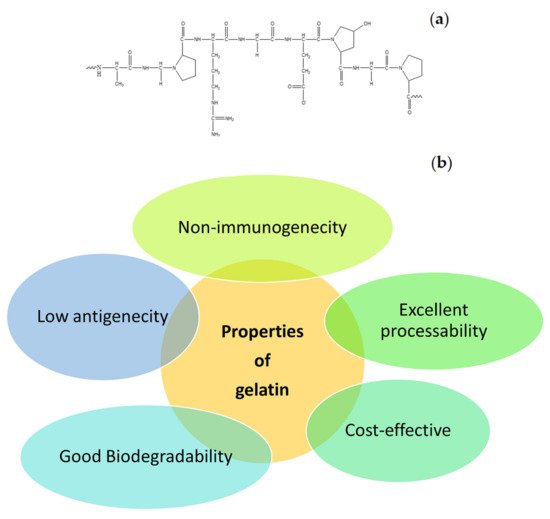
Many natural polymers are frequently used in the formulation of wound dressings. These polymers include gelatin, cellulose, alginate, collagen, elastin, chitosan, chitin, dextran, etc. The common interesting properties of natural polymers are good biocompatibility and biodegradation, non-toxicity, non-immunogenicity, and affordability. In addition, some of the natural polymers exhibit strong attachment to injured tissues and stimulate blood coagulation, accelerate the wound healing process, and induce skin regeneration [12]. Gelatin is one of the biopolymers that is commonly utilized in the design of wound dressings. It is also utilized for biomedical and pharmaceutical applications [15][16]. The molecular structure of gelatin is shown in Figure 2 a. It is a natural mimic of the extracellular matrix (ECM) of human tissues and organs. It is broadly utilized in the field of tissue engineering [17]. The properties of gelatin that have been attracting the attention of most biomedical researchers include excellent biocompatibility, good biodegradability, cell-interactivity, non-immunogenicity, as well as its excellent processability, ready availability, and cost-effectiveness ( Figure 2 b) [18]. The pretty low antigenicity of gelatin also makes it a well-established biopolymer used in numerous biological applications. However, gelatin is a hydrophilic protein, and crosslinking is normally required to enhance its mechanical performance and stability, making gelatin materials insoluble in biological environments [19]. Numerous gelatin crosslinking procedures are available, such as enzymatic using transglutaminase, or chemical using fructose, diepoxy, genipin, dextran dialdehyde, formaldehyde, diisocyanates, glutaraldehyde, or carbodiimides [13].
In various studies, gelatin biopolymers were designed as films, gels, powders, or scaffolds for haemorrhage control in numerous surgical methods [20]. Porous gelatin matrices absorb wound exudates and maintain moisture, thus promoting the wound healing process. Gelatin-based dressings act as porous materials for cell migration and offer mechanical and structural support for the development of new tissue [21]. Although gelatin is a promising biopolymer employed as a wound dressing material, it has no antibacterial efficacy to prevent wound infections or bacterial invasion of the wound [22]. It is combined with other polymers to produce hybrid polymers with superior antibacterial effects.
Gelatin-based scaffolds can be loaded with various antimicrobial agents, such as metal-based nanoparticles, antibiotics, phytochemicals (e.g., curcumin), plant extracts (e.g., Aloe vera), etc., to overcome their poor bactericidal effects [22]. To the best of our knowledge, only two gelatin-based wound dressing materials are commercially available: Gelfoam and Surgifoam. Gelfoam and Surgifoam are composed of porcine gelatin and collagen. Gelfoam and Surgifoam are in the form of compressed sponge and sponge, respectively [23][24]. These commercially available gelatin dressings demonstrate outstanding hemostatic effects. Hence, they are very suitable for bleeding wounds. However, they suffer from some shortcomings, including non-elasticity, etc. [24].
3. Gelatin-Based Hybrid Wound Dressings
Hydrogels are polymeric materials with a good hydrophilic composition that enables their high retention of a significant quantity of water and other biological fluids within their three-dimensional network ( Figure 3 ) [25]. They can be modified for enhanced stability or degradation in the event of contact with biological fluids over an extended period. These polymeric materials have been used in wound healing applications due to their biodegradation, biocompatibility, porosity, ability to encapsulate and release bioactive agents, flexibility, and high-water content [26]. The other advantages of polymer-based hydrogels that have attracted a lot of attention among biomedical researchers in the field of wound management include patient compliance, accelerated wound healing mechanism, the high adsorption capacity of biological fluids which provide moisture to the wound bed, their capability to protect the wound from microorganisms, and specific environmental stimuli-responsiveness (e.g., pH, temperature, and ionic strength). The environmental stimuli-responsive nature of the wound dressings promotes drug release into the infected wound area in a sustained profile, thereby reducing the dosing frequency [27][28]. Several researchers have reported gelatin-based hybrid hydrogels ( Table 1 ).
| Types of Wound Dressings | Polymers Combined with Gelatin | Loaded Bioactive Agents | Outcomes | References |
|---|---|---|---|---|
| Hydrogels | HA | Recombinant thrombomodulin | High swelling capacity and accelerated diabetic wound closure. | [29] |
| Hydrogels | Oxidized Starch | _ | Good cytocompatibility and fast wound healing mechanism with less scar development. | [30] |
| Hydrogels | Pluronic | Nanocurcumin | Accelerated burn wound reduction. | [31] |
| Hydrogels | Gellan | Tannic acid | Superior antimicrobial activity and fast full-thickness wound healing. | [32] |
| Hydrogels | PEG | ASCs | Non-toxicity on skin cells and fast wound contraction. | [33] |
| Hydrogels | Bacterial cellulose | Curcumin | Good mechanical properties and controlled drug release. | [34] |
| Hydrogels | Bacterial cellulose | Methylene blue | Good mechanical performance and high swelling capacity. | [35] |
| Hydrogels | PEG and CMC | _ | Excellent swelling behavior | [36] |
| Hydrogels | PVA and chitosan | _ | Excellent mechanical properties and good hemostatic effects. | [37] |
On the other hand, membranes display similar properties as film wound dressings. The advantages of polymer-based membranes in wound dressing include their ability to absorb excess exudates, maintain appropriate moisture for the wound healing process, retain biological fluids under pressure, do not require frequent dressing changes, reduces the disruption of the wound bed, and present potential cleaning activity [38]. Furthermore, membranes demonstrate good mechanical properties, such as softness, comfortability, flexibility, and stretchability [38].
Jinno et al. , performed comparison studies of bFGF-loaded gelatin-collagen sponges and collagen sponges in wound healing application. The in vivo wound healing studies using full-thickness skin lesions in rats demonstrated that there were enhancement observed in the dermis-like tissue area, the neoepithelial length, and the angiogenesis rates in the group of animal models treated with bFGF-incorporated sponges compared to collagen sponges and plain gelatin-based hybrid sponges [39].
Rather et al., prepared electrospun gelatin-PCL nanofibers functionalized with cerium oxide (CeO 2) nanoparticles for wound healing applications. The FTIR and XRD data confirmed the successful formulation of nanofibers. The cell proliferation assay of the hybrid nanofibers exhibited high proliferation and viability of 3T3-L1 cells, indicating their good biocompatibility. The gelatin-PCL nanofibers functionalized with cerium oxide nanoparticles showed better scavenging potential when compared to the pristine nanofibers, confirming excellent antioxidant efficacy useful in the inflammatory phase of wound healing [40]. Alishahi et al. , designed glucantime-loaded electrospun hybrid nanofibers that are based on gelatin and PVA/PEO/chitosan for wound care of cutaneous Leishmania wounds. The results from this study showed that 4 and 6 cm 2 of drug-loaded hybrid nanofibers destroyed leishmania promastigotes up to 78% with high cell viability of fibroblast cells, indicating that the scaffolds are promising scaffolds for the management of Leishmania wounds [41].
4. Gelatin-Based Hybrid Wound Dressings vs Traditional Wound Dressing Technology
The currently used traditional dressings include plasters, gauze, cotton wool, tulle, bandages, and lint, which are utilized as primary or secondary dressings to protect injuries from contaminations [42]. The other advantages of traditional wound dressings include their ability to absorb wound exudate, offer a dry environment for the wound, and cushion the wound [42][43]. Gauze products that are formulated from non-woven and woven fibers of rayon, polyesters, and cotton can provide a limited barrier against bacterial invasion. Cotton bandages are usually employed for the retention of light wound dressings, short-stretch compression, and high compression bandages offer continuous compression in venous ulcers. Tulle wound dressings (e.g., Paratulle, and Jelonet) are commercially available and are appropriate for superficial clean injury [3]. Although traditional wound dressing products exhibit these interesting advantages, they suffer from several limitations. The disadvantages of traditional dressings include their inability to provide moisture to the wound bed for accelerated wound healing and the capability to cause further skin damage or pain during removal resulting from their high adherent when used in high exuding wounds [44]. They display poor vapor transmission, cause bleeding, and harm the newly formed epithelium during removal. The leakage of wound exudates from traditional wound dressings promotes bacterial invasion [45].
The gelatin-based hybrid wound dressings can be used as ideal dressings to replace the traditional wound dressing products because of their interesting features when compared to the traditional dressings. Gelatin hybrid dressings provide a moist environment for injuries to recover quickly. The suitably moist environment that is offered by gelatin hybrid dressings is due to their moderate WVTR. Interestingly, gelatin-based wound dressings can be loaded with various types of bioactive agents (e.g., antibiotics, nanoparticles, microspheres, antioxidants, etc.) to improve their biological activities and speed up the wound healing process that is essential in the treatment of chronic wounds. Gelatin-based hybrid wound dressings also display good mechanical properties, excellent biocompatibility, non-toxicity, good biodegradation, high porosity, and good absorption and swelling capacity. Nevertheless, gelatin dressings suffer from poor antibacterial activity that is overcome by encapsulating selected antimicrobial agents (such as ciprofloxacin, essential oils, and metal-based nanoparticles) into them. Gelatin-based hybrid wound dressings demonstrate many distinct advantages when compared to the traditional dressings, making them promising scaffolds for the treatment of chronic and high exuding wounds.
This entry is adapted from the peer-reviewed paper 10.3390/polym13172959
References
- Majd, S.A.; Khorasga, M.R.; Moshtaghian, S.J.; Talebi, A.; Khezri, M. Application of Chitosan/PVA Nanofiber as a potential wound dressing for streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 92, 1162–1168.
- Grip, J.; Engstand, R.E.; Skjaeveland, I.; Skalko-Basnet, N.; Isaksoon, J.; Basnet, P.; Holsaeter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280.
- Dhivya, S.; Padma, V.V.; Santhin, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28.
- Boateng, J.; Matthews, K.; Steven, H.; Eccleston, G. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923.
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615.
- Gaspar-pintiliescu, A.; Stanciuc, A.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865.
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42.
- Chronic Wound Care Market. Available online: https://www.fortunebusinessinsights.com/industry-reports/chronic-wound-care-market-100222 (accessed on 26 April 2021).
- Zou, F.; Sun, X.; Wang, X. Elastic, hydrophilic and biodegradable poly (1, 8-octanediol-co-citric acid)/polylactic acid nano fi brous membranes for potential wound dressing applications. Polym. Degrad. Stab. 2019, 166, 163–173.
- Huang, T.; Wang, G.; Tseng, C.; Su, W. Epidermal cells differentiated from stem cells from human exfoliated deciduous teeth and seeded onto polyvinyl alcohol/silk fi broin nano fiber dressings accelerate wound repair. Mater. Sci. Eng. C 2019, 104, 109986.
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Ramakhrishna, S.; Hameed, M.; Khenoussi, N. Enhanced antibacterial activity of PEO-chitosan nanofibers with potential application in burn infection management. Int. J. Biol. Macromol. 2019, 135, 1222–1236.
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art. Curr. Drug Targets 2018, 19, 527–550.
- Dias, J.R.; Baptista-silva, S.; de Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L.; Bartolo, P.J.; Granja, P.L. In situ crosslinked electrospun gelatin nano fi bers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173.
- Wiwatwongwana, F.; Surin, P. In Vitro Degradation of Gelatin/Carboxymethylcellulose Scaffolds for Skin Tissue Regeneretion. Chem. Eng. Trans. 2019, 74, 1555–1560.
- Rujitanaroj, P.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymers 2008, 49, 4723–4732.
- Kang, M.G.; Lee, M.Y.; Cha, J.M.; Lee, J.K.; Lee, S.C.; Kim, J.; Hwang, Y.S.; Bae, H. Nanogels derived from fish gelatin: Application to drug delivery system. Mar. Drugs. 2019, 17, 246.
- Naghibzadeh, M.; Firoozi, S.; Nodoushan, F.S.; Adabi, M.; Khoradmehr, A.; Fesahat, F.; Esnaashari, S.S.; Khosravani, M.; Adabi, M.; Tavakol, S.; et al. Application of eletrospun gelatin in tissue engineering. Biointerface Res. Appl. Chem. 2018, 8, 3048–3052.
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nano fi ber mats for postoperative surgical wounds. Mater. Sci. Eng. C 2016, 58, 242–253.
- Ko, J.H.; Yin, H.; An, J.; Chung, D.J. Characterization of Cross-linked Gelatin Nanofibers through Electrospinning. Macromol. Biosci. 2010, 18, 137–143.
- Agrawal, P.; Soni, A.; Mittal, G.; Bhatnagar, A. Role of polymeric biomaterials as wound healing agents. Int. J. Low. Extrem. Wounds 2014, 13, 180–190.
- Pham-Nguyen, O.-V.; Shin, J.U.; Yoo, H.S. Biomaterials Science mesenchymal stem cells and gelatin nano fi bers for the treatment of full-thickness wounds. Biomater. Res. 2020, 8, 4535.
- Xu, X.; Zhou, M. Antimicrobial Gelatin Nanofibers Containing Silver Nanoparticles. Fibers Polym. 2008, 9, 685–690.
- Schonauer, C.; Tessitore, E.; Barbagallo, G.; Albanese, V.; Moraci, A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur. Spine J. 2004, 13, 89–96.
- Sabel, M.; Stummer, W. The use of local agents: Surgicel and Surgifoam. Eur. Spin J. 2004, 13, 97–101.
- Alven, S.; Aderibigbe, B. Combination Therapy Strategies for the Treatment of Malaria. Molecules 2019, 24, 3601.
- Ajovalasit, A.; Sabatino, M.A.; Todaro, S.; Alessi, S.; Giacomazza, D.; Picone, P.; Carlo, M.D.; Dispenza, C. Xyloglucan-based hydrogel films for wound dressing: Structure-property relationships. Carbohydr. Polym. 2018, 179, 262–272.
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 98, 217–233.
- Wang, N.; Xiao, W.; Niu, B.; Duan, W.; Zhou, L.; Zheng, Y. Highly efficient adsorption of fluoroquinolone antibiotics using chitosan derived granular hydrogel with 3D structure. J. Mol. Liq. 2019, 281, 307–314.
- Hsu, Y.; Liu, K.; Yeh, H.; Lin, H.; Wu, H.; Tsai, J. Sustained release of recombinant thrombomodulin from cross-linked gelatin/hyaluronic acid hydrogels potentiate wound healing in diabetic mice. Eur. J. Pharm. Biopharm. 2019, 135, 61–71.
- Mao, Q.; Hoffmann, O.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Xei, R. Self-contracting oxidized starch/gelatin hydrogel for noninvasive wound closure and wound healing. Mater. Des. 2020, 194, 108916.
- Dang, L.E.H.; Huynh, N.T.; Pham, N.O.; Nguyen, C.T.; Vu, M.T.; Dinh, V.T.; Le, V.T.; Tran, N.Q. Injectable nanocurcumin-dispersed gelatin—Pluronic nanocomposite hydrogel platform for burn wound treatment. Bull. Mater. Sci. 2019, 42, 71.
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775.
- Dong, Y.; Sigen, A.; Rodrigues, M.; Li, X.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.; Wang, W.; Gurther, G.C. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv. Funct. Mater. 2017, 27, 1606619.
- Khamrai, M.; Banerjee, S.; Paul, S.; Samanta, S.; Kundu, P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953.
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91.
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514.
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434.
- Benskin, L.L. Evidence for Polymeric Membrane Dressings as a Unique Dressing Subcategory, Using Pressure Ulcers as an Example. Adv. Wound Care 2018, 7, 419–426.
- Jinno, C.; Morimoto, N.; Ito, R.; Sukamoto, M.; Ogino, S.; Taira, T.; Suzuki, S. A Comparison of Conventional Collagen Sponge and Collagen-Gelatin Sponge in Wound Healing. BioMed Res. Int. 2016, 2016, 4567146.
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Bioactive Materials Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fi bers for wound healing application. Bioact. Mater. 2018, 3, 201–211.
- Alishahi, M.; Khorram, M.; Asgari, Q.; Davani, F.; Goudarzi, F.; Emami, A.; Arastehfar, A.; Zomorodian, K. Glucantime-loaded electrospun core-shell nano fi bers composed of poly (ethylene oxide)/gelatin-poly (vinyl alcohol)/chitosan as dressing for cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 163, 288–297.
- Nešović, K.; Janković, A.; Radetić, T.; Vukašinović-Sekulić, M.; Kojić, V.; Živković, L.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur. Polym. J. 2019, 121, 109257.
- Alven, S.; Aderibigbe, B.A. Hyaluronic Acid-Based Scaffolds as Potential Bioactive Wound Dressings. Polymers 2021, 13, 2102.
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–10.
- Sezer, A.D.; Cevher, E. Biopolymers as wound healing materials: Challenges and new strategies. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011; pp. 383–414.
