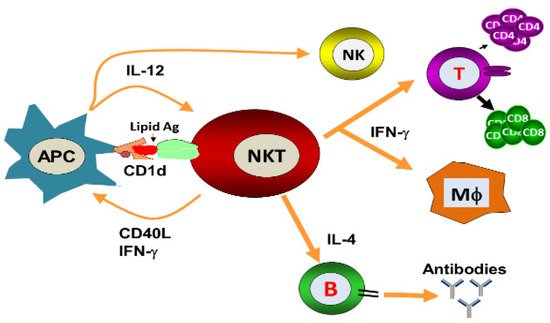
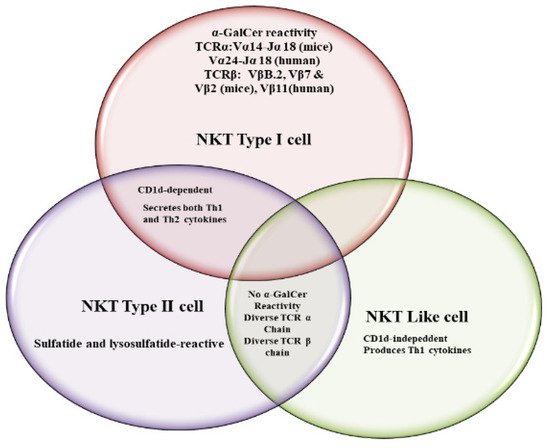
NKT cells have antiviral potential against hepatitis B virus (HBV), respiratory syncytial virus (RSV), encephalomyocarditis virus (EMCV), Herpes simplex virus-1 (HSV-1), coxsackievirus B (CVB), lymphocytic choriomeningitis virus (LCMV), influenza A virus (IAV) and murine cytomegalovirus (MCMV) [
25,
26,
27,
28,
29,
30]. They constitute an important arm of the innate immune response against viruses and can also regulate the adaptive immune responses by modulating the antigen-presenting cells (APCs). NKT cells exert the direct cytolytic effects and restrict the replication of viruses. Moreover, they can indirectly induce an antiviral state through the secretion of important cytokines. α-GalCer-activated NKT cells reduced the replication of murine cytomegalovirus [
30]. Likewise, α-GalCer administration protected the mice against viral encephalomyocarditis [
27]. Wu et al. showed that α-GalCer protected the mice against coxsackievirus B3 (CVB3)-induced myocarditis [
31]. Johnson et al. demonstrated that NKT cell activation has been shown to induce the expansion of Cytotoxic T lymphocytes (CTLs) response and enhance the immune response against RSV [
32]. The activation of the NKT cell resulted in the reduction of the viral load in the pancreas of LCMV-infected mice and this effect was mediated through the secretion of type I interferon [
28]. Whereas, α-GalCer-induced activation of NKT cells against HBV was shown to be mediated by IFN-α/β and IFN-γ secretion [
25]. However, the antiviral effect of NKT cells against the IAV is mediated through the activation of the innate immune responses [
29]. Type I NKT cells exhibited a critical role against IAV infection by increasing IAV-specific CD8
+ T cell response and viral clearance [
33]. Singh et al. showed that cytokines produced by iNKT cells were associated with non-progressive HIV-I infection and patients had a lower viral load in their plasma [
34]. There have been many studies that support the role of NKT cells against viral infections in humans. For example, some patients with mutated adaptor protein SAP (signaling lymphocyte activation molecule-associated protein) were found to be deficient in invariant iNKT cells [
35]. These patients have been found to be susceptible to EBV infection. In an another study, patients mutated in the X-linked inhibitor of the apoptosis protein (XIAP) showed the selective reduction in iNKT cell numbers without affecting B cells, T cells and NK cells [
36]. The Wiskott−Aldrich syndrome (WAS), a primary immune deficiency disease in humans, has been shown to be associated with an iNKT cell deficiency that impaired the functioning of the innate and adaptive immune systems and therefore predisposed the patients to viral infections [
37]. The NKT cells play a very important role in linking the innate and adaptive immunity, their deficiency causes severe immunodeficiency in those persons for whom vaccination with attenuated pathogens is a serious challenge. This was evident from a report that a girl with impaired iNKT cell number and function developed severe respiratory distress after vaccination with the attenuated varicella-zoster virus (VZV) [
38].
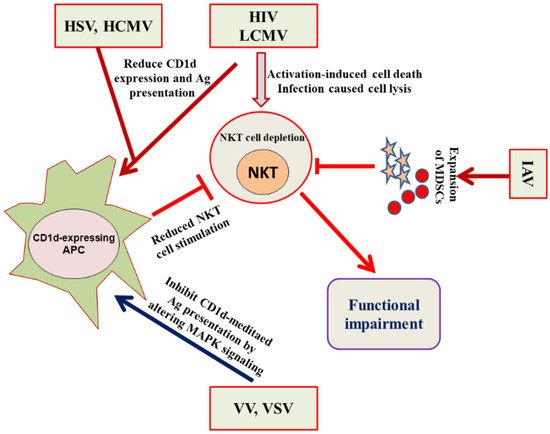
NKT cells have been actively involved in immune responses against HSV-1 and HSV-2 as NKT cell-deficient mice showed severe HSV-1 infection and impaired viral clearance [
39]. The immune evasion strategy of HSV includes the inhibition of NKT cell recognition by curbing the CD1d recycling from the late endosomal compartments to the cell surface [
40]. The protective role of NKT cells against RSV was evident from the fact that Cd1d
−/− mice infected with RSV showed a poor ability to clear the viral load [
39]. MCMV is considered a study model for human cytomegalovirus (HCMV). The iNKT cell activation by MCMV cells requires the involvement of IL-12 and type I interferon, but was independent of CD1d [
41]. In an another study, a role of iNKT cells and CD1d has been suggested to counter the MCMV-induced suppression of hematopoiesis in mice [
42]. The CD1d-dependent activation of NKT cells plays an important role in resisting EMCV infection because Cd1d
−/− mice demonstrated more brutal paralysis due to an acute cytopathic effect of EMCV on neuronal cells [
27]. The iNKT cells also played an important role in protecting against IAV because iNKT cell-deficient mice showed more severe bronchopneumonia. The adoptive transfer of iNKT cells before the infection reversed the effect of IAV-induced bronchopneumonia [
43].
The NKT cells are found in the highest numbers in the livers of mice and therefore are supposed to play a very important role in understanding the pathology of liver diseases [
44]. The activation of NKT cells had a protective role against HBV infection [
25,
45]. The absence of NKT cells or CD1d in mice resulted in diminished HBV-specific T and B cell responses with delayed viral clearance [
46]. Toll-like receptor (TLR) ligands have been shown to activate iNKT cells [
47]. Viruses altered the antigen presentation by CD1d through the activation of pattern recognition receptors, such as TLRs. The endosomal TLRs (TLR 3, 7, 8, and 9) are involved in detecting viruses by recognizing the nucleic acid structures [
48]. TLR-mediated recognition of viruses may lead to altered CD1d-mediated antigen presentation, thereby affecting the activation of iNKT cells. The role of NKT cells has been extensively reported against viral infections in pigs. The percentage of iNKT cells were increased in the blood, lymph node and broncho-alveolar lavage of pigs upon IAV infection [
49]. Renukaradhya et al. demonstrated the role of iNKT cells in the regulation of airway hyper-reactivity [
50]. The intranasal administration of NKT cell ligand ameliorated H1N1 IAV infection in piglets [
51], while the adjuvant effect of α-GalCer potentiated the immune response of the inactivated HIN1 influenza virus in pigs [
52].