Following fertilization, in the mammalian embryo, a series of programmed cell divisions occur whereby the arising cells progressively acquire their own cellular and molecular identity, and totipotency narrows until when pluripotency is achieved. The path towards pluripotency involves transcriptome modulation, remodeling of the chromatin epigenetic landscape to which external modulators contribute. Both human and mouse embryos are a source of different types of pluripotent stem cells whose characteristics can be captured and maintained in vitro.
- peri-implantation embryo
- EGA
- lineage specification
- pluripotent stem cells
- pluripotency transcriptional networks
- epigenetic
- DNA methylation and histone modification
- X chromosome inactivation
- non-coding RNAs
1. Introduction
The ability of a cell to differentiate and give rise to different specialized cell types represents the cell potency. Thus, depending on a cell’s differentiation potential, potency spans from totipotency, pluri-, multi-, oligo- or uni-potency [1][2]. The fusion of two highly differentiated cells, an oocyte with a spermatozoon, gives rise to the zygote, a single totipotent cell, which has the capability to develop into an entire, fully functional organism. Then, as development proceeds, totipotency becomes restricted at stages that vary among species.
In the mouse, following the first cell division, the blastomeres of 2-cell stage embryos may not be equally totipotent. Unequal segregation of the zygote cytoplasmic components [3] may influence totipotency continuity in the blastomere pairs. Then, embryonic cells evolve towards pluripotency while the embryos undergo through cellular events that occur at specific time points after fertilization. At 2.5 days post coitum (dpc), 8-cell stage embryos undergo compaction, followed by morula cavitation and blastocyst formation (3.5 dpc), the latter constituted of an outer single-layered epithelium, the trophectoderm (TE) and an inner cell mass (ICM) facing a fluid-filled cavity (first lineage specification) ( Figure 1 ). While the TE forms the fetal component of the placenta, the ICM, initially made of common progenitor cells [4], gives rise, through a second lineage specification, to the epiblast (EPI) and the primitive endoderm (PrE) ( Figure 1 ) [5]. During this time window, EPI cells become pluripotent, i.e., able to develop into ectoderm, mesoderm and endoderm (the three germ layers), to the germ line and to the extraembryonic ectoderm and mesoderm. At 4.5 dpc, following hatching from the zona pellucida, the mature blastocyst implants into the endometrium and gastrulation will then follow at 6.5 dpc.
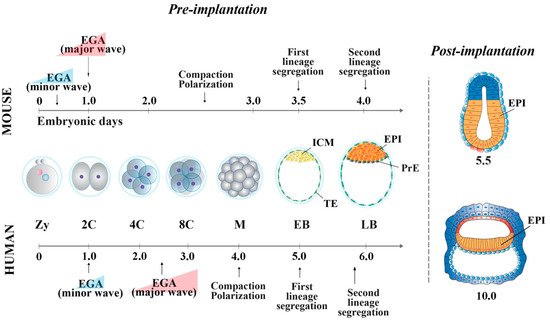
2. Pluripotency features of Mouse and Human Peri-Implantation Embryos
From the morula stage, lineage specification and differentiation are accompanied by a decrease of global cell potency, the latter achieved through a precise spatio-temporal activation of key genes [14][15]. At this stage, the first lineage specification is determined by several regulatory pathways [16][17][18][19][20]. In outer cells, the inactivity of the Hippo pathway allows the expression of Cdx2 and Gata-3 , which together with Notch , Eomes and Elf5 contribute to the establishment of TE fate [21][22]51,[23][24][25][26][27][28]. In the inner cells, the Hippo pathway is activated, allowing transcription of ICM-specific genes (e.g., Oct-4 , Nanog , Sox2 and Esrrb) [29]. Mouse ICM is composed by heterogeneous cells, which co-express different levels of lineage-associated factors, such as Gata-6 (PrE) and Nanog (EPI) [30][31].
At the blastocyst stage, cells of the ICM undergo a second lineage specification determined by the FGF/FGF-receptor signaling, mediated via MEK/ERK [32][33]. Gata-6 and FGF/ERK induce PrE fate [24][34][35], whereas Gata-4 and Sox17 are involved in its maintenance [34][36]. EPI cells are characterized by the expression of Oct-4, Nanog, Sox2, Kfl2, Klf17 and Esrrb, all involved in pluripotency maintenance [21][37][24][38]. Specifically, the establishment and maintenance of the pluripotency state is characterized by a well-defined “pluripotency gene regulatory network” (PGRN) and by a strong cooperation of transcription and epigenetic factors that act in synergy [39][40]. The PGRN is a very highly interconnected system, in which Oct-4, Nanog and Sox2 represent the central functional core. Among this triad of genes, Oct-4 is at the top of the pluripotency regulatory hierarchy, being essential to reach and maintain pluripotency [41][42][43].
In human embryos, at the compacted morula stage (4 dpf), both specific TE determinants and ICM-related genes are expressed to determine the first lineage specification [66]. The lineage-specific transcripts become mutually exclusive only at the early blastocyst stage (5 dpf). However, it has been shown that 5 dpf TE cells still retain the ability to form ICM cells [92], and, conversely, isolated ICMs can also generate TE cells [93], indicating that cells at this stage of development are not yet fully committed. The specification of the cell lineages does not seem to occur through a stepwise process, as for the mouse [66], with transcriptional differences being detected at 5 dpf, once the blastocyst is formed. Additionally, it is unclear whether Hippo pathway determines the first cell fate decision [55,94]. GATA-3 and CDX2 are also involved in TE cell determination [53,56,95]. Then, a second lineage specification occurs, where human EPI cells display the expression of NANOG, OCT-4 and SOX2, all required for pluripotency maintenance, and of KLF17 [24][38][44]. GATA-6, initially broadly expressed in the early blastocysts, is involved in PrE fate induction [27][34], together with SOX17 and GATA-4. These latter two are expressed later and are restricted to the PrE, where they are required for its maintenance [24][34].
During the early phases of development, molecular modifications, e.g., DNA methylation, histone methylation and acetylation, and, for female embryos, the progressive silencing of X-linked genes for dosage compensation, determine specific epigenetic landscapes, which contribute to the progressive acquisition of pluripotency.
3. Pluripotency Features of Stem Cells In Vitro
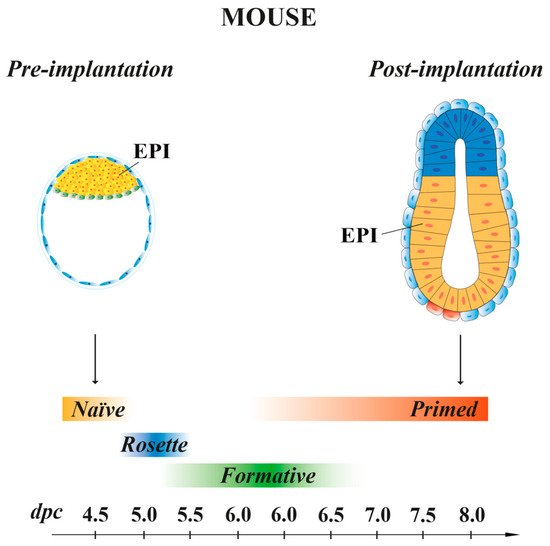
SCs, isolated either from embryos at different stages of pre- and early post-implantation development or from PSC lines, can be maintained in vitro applying self-renewal culture conditions [45][46] and possess cellular and molecular features that mirror different pluripotency states, defined as extended, naïve, intermediate and primed. They are characterized by distinguishable colony morphology, growth factor requirement, energetic metabolism, molecular signatures and, in the female cell lines, X inactivation status [40][47][48].
Mouse ESCs retain the same molecular and transcriptional features of the EPI cells present at 4.5 dpc pre-implantation embryo stage [49], with a pluripotency characteristic called “naïve” [47] (Figure 2). When injected in early pre-implantation embryos, naïve mESCs contribute to all somatic lineages and to germline, indicative of their pluripotency in vivo [50]. Mouse ESCs are in an unstable balance between pluripotency and differentiation signals, which support self-renewal (maintained by LIF) or promote differentiation (induced by FGF). However, the addition of exogenous LIF favors self-renewal at the expense of differentiation [51]. In recent years, SCs, derived from either 5–6.5 dpc mouse embryos or naïve ESCs, have been shown to possess intermediate states of pluripotency between naïve and primed. These transition pluripotency states are defined as “poised” [52], “rosette” [53] or “formative” [54][55][56][57] (Figure 2). These cells, while downregulating the naïve transcriptional program, begin to acquire the competence for multi-lineage differentiation, although they do not yet express lineage-associated markers. Mouse EpiSCs, derived from the EPI of post-implantation embryos at 6.25–8 dpc, are characterized by a pluripotency status named “primed”. As naïve mESCs, primed mEpiSCs display unlimited potential to self-renewal and differentiate into the three germ layers in vitro, but they are limited in their pluripotency in vivo, as they cannot give rise to blastocyst chimeras [10][58][59].
This entry is adapted from the peer-reviewed paper 10.3390/cells10082049
References
- Denker, H.W. Stem cell terminology and ‘synthetic’ embryos: A new debate on totipotency, omnipotency, and pluripotency and how it relates to recent experimental data. Cells Tissues Organs 2014, 199, 221–227.
- Singh, V.K.; Saini, A.; Kalsan, M.; Kumar, N.; Chandra, R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In silico Diagnostics. Front. Cell Dev. Biol. 2016, 4, 134.
- Casser, E.; Israel, S.; Boiani, M. Multiplying embryos: Experimental monozygotic polyembryony in mammals and its uses. Int. J. Dev. Biol. 2019, 63, 143–155.
- Saiz, N.; Williams, K.M.; Seshan, V.E.; Hadjantonakis, A.K. Asynchronous fate decisions by single cells collectively ensure consistent lineage composition in the mouse blastocyst. Nat. Commun. 2016, 7, 13463.
- Gardner, R.L.; Rossant, J. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J. Embryol. Exp. Morphol. 1979, 52, 141–152.
- Shahbazi, M.N.; Siggia, E.D.; Zernicka-Goetz, M. Self-organization of stem cells into embryos: A window on early mammalian development. Science 2019, 364, 948–951.
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638.
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156.
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147.
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199.
- Brons, I.G.; Smithers, L.E.; Trotter, M.W.; Rugg-Gunn, P.; Sun, B.; Chuva de Sousa Lopes, S.M.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007, 448, 191–195.
- Osorno, R.; Tsakiridis, A.; Wong, F.; Cambray, N.; Economou, C.; Wilkie, R.; Blin, G.; Scotting, P.J.; Chambers, I.; Wilson, V. The developmental dismantling of pluripotency is reversed by ectopic Oct4 expression. Development 2012, 139, 2288–2298.
- Riveiro, A.R.; Brickman, J.M. From pluripotency to totipotency: An experimentalist’s guide to cellular potency. Development 2020, 147, dev189845.
- Ben-Tabou de-Leon, S.; Davidson, E.H. Gene regulation: Gene control network in development. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 191.
- Mallo, M.; Alonso, C.R. The regulation of Hox gene expression during animal development. Development 2013, 140, 3951–3963.
- Yagi, R.; Kohn, M.J.; Karavanova, I.; Kaneko, K.J.; Vullhorst, D.; DePamphilis, M.L.; Buonanno, A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development 2007, 134, 3827–3836.
- Nishioka, N.; Yamamoto, S.; Kiyonari, H.; Sato, H.; Sawada, A.; Ota, M.; Nakao, K.; Sasaki, H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 2008, 125, 270–283.
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 2009, 16, 398–410.
- Anani, S.; Bhat, S.; Honma-Yamanaka, N.; Krawchuk, D.; Yamanaka, Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development 2014, 141, 2813–2824.
- Hirate, Y.; Hirahara, S.; Inoue, K.; Kiyonari, H.; Niwa, H.; Sasaki, H. Par-aPKC-dependent and -independent mechanisms cooperatively control cell polarity, Hippo signaling, and cell positioning in 16-cell stage mouse embryos. Dev. Growth Differ. 2015, 57, 544–556.
- Gerri, C.; Menchero, S.; Mahadevaiah, S.K.; Turner, J.M.A.; Niakan, K.K. Human Embryogenesis: A Comparative Perspective. Annu. Rev. Cell Dev. Biol. 2020, 36, 411–440.
- Ng, R.K.; Dean, W.; Dawson, C.; Lucifero, D.; Madeja, Z.; Reik, W.; Hemberger, M. Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat. Cell Biol. 2008, 10, 1280–1290.
- Blakeley, P.; Fogarty, N.M.; del Valle, I.; Wamaitha, S.E.; Hu, T.X.; Elder, K.; Snell, P.; Christie, L.; Robson, P.; Niakan, K.K. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 2015, 142, 3151–3165.
- Strumpf, D.; Mao, C.A.; Yamanaka, Y.; Ralston, A.; Chawengsaksophak, K.; Beck, F.; Rossant, J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 2005, 132, 2093–2102.
- Niakan, K.K.; Eggan, K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev. Biol. 2013, 375, 54–64.
- Ralston, A.; Cox, B.J.; Nishioka, N.; Sasaki, H.; Chea, E.; Rugg-Gunn, P.; Guo, G.; Robson, P.; Draper, J.S.; Rossant, J. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development 2010, 137, 395–403.
- Noli, L.; Dajani, Y.; Capalbo, A.; Bvumbe, J.; Rienzi, L.; Ubaldi, F.M.; Ogilvie, C.; Khalaf, Y.; Ilic, D. Developmental clock compromises human twin model created by embryo splitting. Hum. Reprod. 2015, 30, 2774–2784.
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254.
- Menchero, S.; Rollan, I.; Lopez-Izquierdo, A.; Andreu, M.J.; Sainz de Aja, J.; Kang, M.; Adan, J.; Benedito, R.; Rayon, T.; Hadjantonakis, A.K.; et al. Transitions in cell potency during early mouse development are driven by Notch. Elife 2019, 8, e42930.
- Gharanfoli, S.; Shahverdi, A.; Dalman, A.; Ghaznavi, P.; Alipour, H.; Eftekhari-Yazdi, P. Effect of Maternal Age on Hippo Pathway Related Gene Expressions and Protein Localization Pattern in Human Embryos. Cell J. 2020, 22, 74–80.
- Chazaud, C.; Yamanaka, Y.; Pawson, T.; Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 2006, 10, 615–624.
- Dietrich, J.E.; Hiiragi, T. Stochastic patterning in the mouse pre-implantation embryo. Development 2007, 134, 4219–4231.
- Guo, G.; Huss, M.; Tong, G.Q.; Wang, C.; Sun, L.; Clarke, N.D.; Robson, P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 2010, 18, 675–685.
- Yamanaka, Y.; Lanner, F.; Rossant, J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 2010, 137, 715–724.
- Roode, M.; Blair, K.; Snell, P.; Elder, K.; Marchant, S.; Smith, A.; Nichols, J. Human hypoblast formation is not dependent on FGF signalling. Dev. Biol. 2012, 361, 358–363.
- Petropoulos, S.; Edsgärd, D.; Reinius, B.; Deng, Q.; Panula, S.P.; Codeluppi, S.; Reyes, A.P.; Linnarsson, S.; Sandberg, R.; Lanner, F. Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell 2016, 167, 285.
- Artus, J.; Piliszek, A.; Hadjantonakis, A.K. The primitive endoderm lineage of the mouse blastocyst: Sequential transcription factor activation and regulation of differentiation by Sox17. Dev. Biol. 2011, 350, 393–404.
- Cauffman, G.; De Rycke, M.; Sermon, K.; Liebaers, I.; Van de Velde, H. Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum. Reprod. 2009, 24, 63–70.
- Young, J.C.; Major, A.T.; Miyamoto, Y.; Loveland, K.L.; Jans, D.A. Distinct effects of importin α2 and α4 on Oct3/4 localization and expression in mouse embryonic stem cells. FASEB J. 2011, 25, 3958–3965.
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J.H. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell. Biol. 2016, 17, 155–169.
- Pesce, M.; Anastassiadis, K.; Schöler, H.R. Oct-4: Lessons of totipotency from embryonic stem cells. Cells Tissues Organs 1999, 165, 144–152.
- Jaenisch, R.; Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008, 132, 567–582.
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391.
- De Paepe, C.; Cauffman, G.; Verloes, A.; Sterckx, J.; Devroey, P.; Tournaye, H.; Liebaers, I.; Van de Velde, H. Human trophectoderm cells are not yet committed. Hum. Reprod. 2013, 28, 740–749.
- Guo, S.; Cui, X.; Jiang, X.; Duo, S.; Li, S.; Gao, F.; Wang, H. Tracing the origin of the placental trophoblast cells in mouse embryo development†. Biol. Reprod. 2020, 102, 598–606.
- Royer, C.; Leonavicius, K.; Kip, A.; Fortin, D.; Nandi, K.; Vincent, A.; Jones, C.; Child, T.; Coward, K.; Graham, C.; et al. Establishment of a relationship between blastomere geometry and YAP localisation during compaction. Development 2020, 147, dev189449.
- Chen, L.; Yabuuchi, A.; Eminli, S.; Takeuchi, A.; Lu, C.W.; Hochedlinger, K.; Daley, G.Q. Cross-regulation of the Nanog and Cdx2 promoters. Cell Res. 2009, 19, 1052–1061.
- Hyslop, L.; Stojkovic, M.; Armstrong, L.; Walter, T.; Stojkovic, P.; Przyborski, S.; Herbert, M.; Murdoch, A.; Strachan, T.; Lako, M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells 2005, 23, 1035–1043.
- Boutourlinsky, K.; Allègre, N.; Chazaud, C. Ex Vivo Culture for Preimplantation Mouse Embryo to Analyze Pluripotency. Methods Mol Biol. 2021, 2214, 1–10.
- Nichols, J.; Smith, A. Pluripotency in the embryo and in culture. Cold Spring Harb. Perspect. Biol. 2012, 4, a008128.
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492.
- Devika, A.S.; Wruck, W.; Adjaye, J.; Sudheer, S. The quest for pluripotency: A comparative analysis across mammalian species. Reproduction 2019, 158, R97–R111.
- Boroviak, T.; Loos, R.; Bertone, P.; Smith, A.; Nichols, J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 2014, 16, 516–528.
- Czechanski, A.; Byers, C.; Greenstein, I.; Schrode, N.; Donahue, L.R.; Hadjantonakis, A.K.; Reinholdt, L.G. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat. Protoc. 2014, 9, 559–574.
- Kunath, T.; Saba-El-Leil, M.K.; Almousailleakh, M.; Wray, J.; Meloche, S.; Smith, A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007, 134, 2895–2902.
- Du, P.; Pirouz, M.; Choi, J.; Huebner, A.J.; Clement, K.; Meissner, A.; Hochedlinger, K.; Gregory, R.I. An Intermediate Pluripotent State Controlled by MicroRNAs Is Required for the Naive-to-Primed Stem Cell Transition. Cell Stem Cell 2018, 22, 851–864.
- Neagu, A.; van Genderen, E.; Escudero, I.; Verwegen, L.; Kurek, D.; Lehmann, J.; Stel, J.; Dirks, R.A.M.; van Mierlo, G.; Maas, A.; et al. In vitro capture and characterization of embryonic rosette-stage pluripotency between naive and primed states. Nat. Cell Biol. 2020, 22, 534–545.
- Smith, A. Formative pluripotency: The executive phase in a developmental continuum. Development 2017, 144, 365–373.
- Kinoshita, M.; Smith, A. Pluripotency Deconstructed. Dev. Growth Differ. 2018, 60, 44–52.
- Wang, X.; Xiang, Y.; Yu, Y.; Wang, R.; Zhang, Y.; Xu, Q.; Sun, H.; Zhao, Z.A.; Jiang, X.; Wang, X.; et al. Formative pluripotent stem cells show features of epiblast cells poised for gastrulation. Cell Res. 2021, 31, 526–541.
- Kinoshita, M.; Barber, M.; Mansfield, W.; Cui, Y.; Spindlow, D.; Stirparo, G.G.; Dietmann, S.; Nichols, J.; Smith, A. Capture of Mouse and Human Stem Cells with Features of Formative Pluripotency. Cell Stem Cell 2021, 28, 453–471.
- Guo, G.; Yang, J.; Nichols, J.; Hall, J.S.; Eyres, I.; Mansfield, W.; Smith, A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009, 136, 1063–1069.
- Huang, Y.; Osorno, R.; Tsakiridis, A.; Wilson, V. In Vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2012, 2, 1571–1578.
- O’Leary, T.; Heindryckx, B.; Lierman, S.; van Bruggen, D.; Goeman, J.J.; Vandewoestyne, M.; Deforce, D.; de Sousa Lopes, S.M.; De Sutter, P. Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat. Biotechnol. 2012, 30, 278–282.
- O’Leary, T.; Heindryckx, B.; Lierman, S.; Van der Jeught, M.; Duggal, G.; De Sutter, P.; Chuva de Sousa Lopes, S.M. Derivation of human embryonic stem cells using a post-inner cell mass intermediate. Nat. Protoc. 2013, 8, 254–264.
- De Los Angeles, A.; Ferrari, F.; Xi, R.; Fujiwara, Y.; Benvenisty, N.; Deng, H.; Hochedlinger, K.; Jaenisch, R.; Lee, S.; Leitch, H.G.; et al. Hallmarks of pluripotency. Nature 2015, 525, 469–478.
- De Los Angeles, A. The Pluripotency Continuum and Interspecies Chimeras. Curr. Protoc. Stem Cell Biol. 2019, 50, e87.
- Hanna, J.; Cheng, A.W.; Saha, K.; Kim, J.; Lengner, C.J.; Soldner, F.; Cassady, J.P.; Muffat, J.; Carey, B.W.; Jaenisch, R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA 2010, 107, 9222–9227.
- Takashima, Y.; Guo, G.; Loos, R.; Nichols, J.; Ficz, G.; Krueger, F.; Oxley, D.; Santos, F.; Clarke, J.; Mansfield, W.; et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 2014, 158, 1254–1269.
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L.; et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014, 15, 471–487.
- Chan, Y.S.; Göke, J.; Ng, J.H.; Lu, X.; Gonzales, K.A.; Tan, C.P.; Tng, W.Q.; Hong, Z.Z.; Lim, Y.S.; Ng, H.H. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 2013, 13, 663–675.
- Duggal, G.; Warrier, S.; Ghimire, S.; Broekaert, D.; Van der Jeught, M.; Lierman, S.; Deroo, T.; Peelman, L.; Van Soom, A.; Cornelissen, R.; et al. Alternative Routes to Induce Naïve Pluripotency in Human Embryonic Stem Cells. Stem Cells 2015, 33, 2686–2698.
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286.
- Ware, C.B.; Nelson, A.M.; Mecham, B.; Hesson, J.; Zhou, W.; Jonlin, E.C.; Jimenez-Caliani, A.J.; Deng, X.; Cavanaugh, C.; Cook, S.; et al. Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4484–4489.
