Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Much of the complex medical physics work requires radiation dose delivery, which requires dosimeters to accurately measure complex three-dimensional dose distribution with good spatial resolution. MAGIC-f polymer gel is one of the emerging new dosimeters widely used in medical physics research. The purpose of this study was to present an overview of polymer gel dosimetry, using MAGIC-f gel, including its composition, manufacture, imaging, calibration, and application to medical physics research.
- MAGIC-f gel
- dosimetry
- polymer gel
- irradiation
Note: The entry will be online only after author check and submit it.
1. Introduction
Gel dosimeters are composed of radiation-sensitive chemicals that experience a fundamental change in their properties following ionizing radiation, due to the absorbed dose [1,2]. These features of the polymeric gel dosimeters can store dose distribution information in three dimensions, an additional benefit compared to other dosimeters that provide only one-dimensional dose distribution, such as ionization chambers and films in a point, or two-dimensional dose distribution [2,3]. This benefit is especially important for emerging technologies relating to radiation, where there is a substantial incidence of high-dose gradients [4]. There are various kinds of gel dosimeters that have been discovered so far, but two types, with the names Fricke and polymer gel, have been extensively used. These polymer gels are usually analyzed by using various diagnostic imaging techniques, such as sonography, optical computer tomography (OCT), X-ray computer tomography (X-ray CT), and magnetic resonance imaging (MRI) [5].
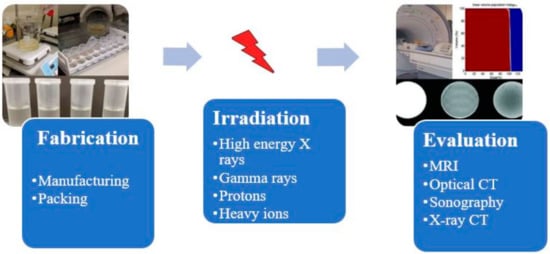
A radiation dosimetry process with MAGIC-f (Methacrylic Ascorbic Acid in Gelatin Caused by Copper-Added Formaldehyde) gel consists of steps involving gel preparation, irradiation with ionizing radiation, taking images of irradiated gels using some imaging techniques and finally analyzing the images to calculate the dose distribution. A typical polymer gel dosimetry process involves three stages known as fabrication, irradiation, and evaluation stages, as shown in Figure 1. Therefore, development process of MAGIC-f gel dosimetry consists of development of all these three processes as well. Thus, in this review, besides the discussion of development trends of MAGIC-f gel from the development of a precursor form of polymer gel such as dye made up of methylene, development of each step of MAGIC-f dosimetry is also discussed. Hence, this review briefly examines the current knowledge of dosimetry using MAGIC-f gel to emphasize its structure, manufacture, imaging, calibration, and application to medical physics research.
2. Development of MAGIC-f Gel
The development trends of MAGIC-f gel can be described from the development of a precursor form of polymer gel, such as dye made up of methylene, to the latest important innovation in the polymer gel dosimetry using MAGIC-f gel. Before the polymer gel dosimeter’s innovation, dosimetry calculations were made only through ion chambers and film, which only gives point dose and two-dimensional dose distribution, respectively [3]. The use of a sensitive gel to irradiation was first proposed by Day and Stein in 1950, where the gel containing dye such as methylene blue gives color changes when subjected to radiation [8]. However, the use of polymer system/gel for radiation dosimetry was first initiated by Alexander et al. in 1954 [9]; Hoecker et al., in 1958, worked on radiation dosimetry using liquid polymer [10]; and Boni. Et al. used polyacrylamide in 1961 [11]. Audet et al. found improvements in measurements of irradiated polyethylene oxide transverse relaxation by NMR [12]. The credit of discovery of the currently used radiation-sensitive polymer gel for radiation dosimetry goes to Gore et al. [13], who investigated the relaxation properties after irradiation of previously developed ferrous sulfate chemical dosimeter by Fricke et al. [14,15], by nuclear NMR. Gore et al. showed that radiation-induced shifts could be quantified by using NMR relaxation steps where irradiation causes iron ions to gain a higher oxidation state, i.e., from ferrous (Fe2+) into ferric (Fe3+) ions [13]. Later, Kennan et al. observed increased relaxation rates with the absorbed dose from the longitudinal relaxation studies of NMR conducted on irradiated N,N′-methylene-bis-acrylamide form of aqueous solution [16].
The polymer gel proposed by Gore et al. is a precursor version of the currently utilized polymer gel dosimeter for radiation dosimetry, which requires significant improvements due to many difficulties encountered during use. The polymer-gel dosimeter is based on radiation-induced polymerization and crosslinking of acrylic monomers [17]. In contrast, Fricke gel is not a polymer-based dosimeter; it is based on radiation-induced oxidation of ferrous ions which modifies NMR relaxation rates. In 1986, Appleby et al.’s research showed that a gel matrix with Fricke dosimetry solutions could be used to obtain spatial three-dimensional dose distribution using magnetic resonance imaging [18]. Further, opposing the claim of previous researchers, failure of irradiated gel dosimeters of Fricke-type to maintain stable distribution of dose in three-dimensional space was shown for the purpose of ion diffusion in the dosimeters that are irradiated [19,20], and several authors’ publications also support this claim. As diffusion was the big problem in the innovation in polymer gel dosimetry, attempts made by Baldock et al. to investigate the Fricke solution with different gelling agents, such as gelatin, agarose, Sephadex, polyvinyl alcohol (PVA), and xylenol orange (XO), where xylenol orange gives the best result, but performance is minimal, and the diffusion cannot be omitted and remain as a problem [21]. Prior to Baldock et al., Tomas et al. also investigated to cope up with diffusion effect, where they proposed to use fast T1 imaging sequence to reduce the acquisition time and, ultimately, diffusion effect; they also found that diffusion can be lowered by adding chelating agents, such as xylenol orange to the gel, and found that diffusion was slower in gelatin gels [22]. Another system of polymer gel dosimetry that goes by the acronym BANANA polymer gel, due to the use of Bis (N,N-methylene-bis-acrylamide), AAm (acrylamide), nitrous oxide, and agarose, was formulated in 1992 by Maryanski et al., without having the diffusion problem [23]. Later, agarose in the BANANA gel was replaced by gelatin to form BANG (Bis, AAm, nitrogen, and aqueous gelatin) gel, which was patented for commercial use [24,25], and its in-house version is known as PAG gel [26]. Various compositions and formulations of polymer gel dosimeter have been studied, which brings significant innovation in polymer gel proposed by Gore et al.
The interest in the development of polymer gel dosimeters with different compositions and formulations continued. The Fricke-type gel dosimeter’s limitation has been addressed by several studies [5,27]; one major limitation of the Fricke-type dosimeter was the atmospheric oxygen inhibiting polymerization, as the radiation-induced polymerization is ineffective in those parts of the dosimeter contaminated by air. Hence, an oxygen-free environment should be maintained to manufacture the gel dosimeter. Fong et al. addressed this issue by developing a polymer gel dosimeter acronym as MAGIC due to its composition (Methacrylic and Ascorbic acid in Copper-Initiated Gelatin) [28]. The MAGIC gel can be manufactured in a normal laboratory environment, as the problem of oxygen inhibition was resolved by binding atmospheric oxygen in an organic complex (free oxygen found in the matrix of gelatin is bound by ascorbic acid to metallic–organic complexes with the help of metal-composed copper sulfate) [29]. Later, other authors proposed different kinds of antioxidants to manufacture such a class of polymer gel known as normoxic polymer gel [30]; as an example, tetrakis (hydroxymethyl) phosphonium chloride (THPC) was proposed by De Deene et al. [31].
Another issue that occurred in polymer gel dosimetry was the melting of the gel samples when kept at average room temperature, which resulted in a lack of knowledge on the dose distribution, hence restricting its use. Fernandes et al. solved this issue in 2008 by adding formaldehyde to the existing MAGIC formulation, which raised the melting point of gel to 69 °C and named it MAGIC-f gel [32]. Formaldehyde was applied to the MAGIC gel to increase the matrix’s stability through the development of the high number of hydrogen bonds between the gelatin and formaldehyde. The fusion point of MAGIC-f gel doubles if 1% of formaldehyde is used [28,32]. Hence, the MAGIC-f gel dosimeter is the latest innovation in the field of three-dimensional radiation dosimetry.
3. MAGIC-Gel Composition and Fabrication
It is important to understand the fundamental chemistry and physics of response before understanding the fabrication process. To understand the fundamental chemistry and physics of response, the composition and benefits of using each element of the composition should be known. A typical polymer gel should have a composition that gives a shape, ingredients that polymerize when subjected to radiation, and ingredients that retain the gel’s polymerization ability. A typical polymer gel consists of five different compositions to use as a dosimeter and give three-dimensional dose distribution when irradiated, as shown in Figure 2. The first two components are water and gelatin, which form the block structure of the dosimeter. Two active ingredients are monomers and crosslinker, which polymerize when exposed to ionizing radiation, i.e., with more ionizing radiation, more polymerizations occur. The last ingredient is oxygen scavenger. Too much free oxygen in a dosimeter makes it inert; thus, an oxygen scavenger is used to remove chemically active dissolved oxygen, which suppresses the radical initiated polymerization.
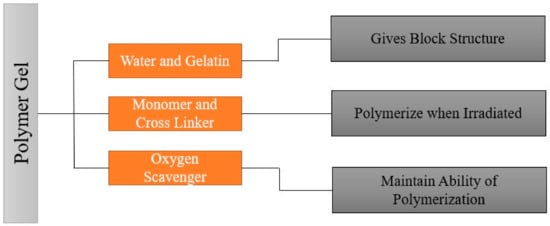
Figure 2. General composition of the polymer gel.
Since 1954, different changes in composition have been made to make the polymer gel more accurate in three-dimensional dose distribution and easy to manufacture, handle, and use. Early polymer gel proposed by Gore et al., consisting of ferrous sulfate, and later used by Appleby et al., consisting of ferrous sulfate dispersed in a gel matrix, could not give a spatially stable distribution of dose because of diffusion of ion [13,18]. After these, several studies used not only ferrous solutions with various gelling agents, such as gelatin, agarose, Sephadex, and polyvinyl alcohol (PVA), but also chelating agents, such as xylenol orange (XO), to reduce ion diffusion [21]. However, there was an issue in manufacture because the polymerization can be disturbed due to the oxygen presence; hence, hypoxic environment is necessary to manufacture it. To address this issue, Fong et al. developed a novel polymer gel, acronym as MAGIC gel, which is formed by mixing methacrylate-based materials, ascorbic acid, and salt copper [28]. Ascorbato–copper complex provides oxygen absorption, allowing for the manufacturing of polymer gels under standard atmospheric conditions in 2001 [33]. Another issue was the melting of samples when kept at room temperature, creating a problem for its use and handle. Fernandes et al. gave the solution to this issue in 2008 by incorporating formaldehyde to the earlier MAGIC formulation, raising the melting point of gel to 69 °C, and labeling the new gel as MAGIC-f gel [32]. The formaldehyde improved the stability of the matrix as a result of the high number of hydrogen bonds formed between the formaldehyde and gelatin. Note that the MAGIC-f gel fusion point doubles if 1% of formaldehyde is used [28,33]. After 2008, the MAGIC-f gel with the atomic and chemical composition shown in Figure 3 was proposed and used by most of the studies, including that by T. Marques et al. [34]. In the atomic composition (w/w), H (10.33%), O (62.68%), C (23.52%), N (2.52%), and others (0.81%) are used. Chemical composition: w/w of 82.7% of Milli-q water (water that has been purified using resin filters and deionized to a high degree by a water purification system), 8.4% of gelatin, 0.02% of copper, 0.03% of ascorbic acid, 5.9% of methacrylic acid, and 1% of formaldehyde. Although the composition proposed by Fernandes et al. has been adopted in several studies, a few studies done by Pavoni et al. increased the composition of formaldehyde in the gel. They used a greater weight of formaldehyde in the gel mixture, i.e., 3.3% by weight in mixture, where most of the studies just used 1% by weight in gel mixture [35,36].
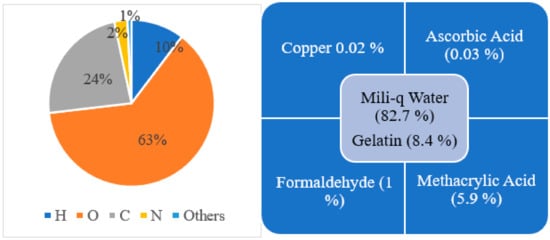
Figure 3. Atomic (left side) and chemical (right side) composition for MAGIC-f gel.
4. Irradiation of MAGIC-f Gel
The dosimetry analysis using MAGIC-f gel is based on the chemical reaction induced by irradiation. The study of three-dimensional dose distribution in MAGIC-f gel is performed by using different radiation sources. As polymer gel dosimetry has been studied and used in various clinical applications since the 1950s; most studies use radiation of gamma rays and high-energy X-ray photons from different clinical linear accelerator sources [3]. Another form of irradiation is protons and heavy ions, but due to the saturation effect, the dosimetric reaction is lower than that from using gamma and X-rays [37,38,39,40,41,42,43]. Most of the studies on MAGIC-f gel also used gamma rays and high-energy X-ray photons from different clinical linear accelerator sources; however, only one study by Pianoschi et al. used an electron beam [44].
Figure 4 summarizes the basic chemical reaction on the MAGIC-f gel dosimeter after irradiation. In this reaction, several different free radicals are produced as a result of radiolysis of water molecule. The free radicals then react with the vinyl group (double bond between carbon atoms C = C) of the monomers, i.e., methacrylic acid. Moreover, there will be successive propagation and crosslinking reaction until all the radicals are consumed by the chain transfer reaction to form the polymer. This reaction is directly proportionate to the amount of the irradiation on the MAGIC-f gel dosimeter.
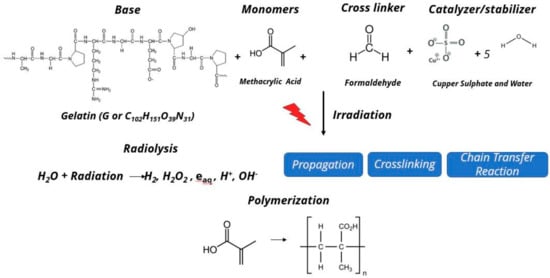
Figure 4. Chemical reaction after irradiation on MAGIC-f gel.
This entry is adapted from the peer-reviewed paper 10.3390/app11177783
This entry is offline, you can click here to edit this entry!