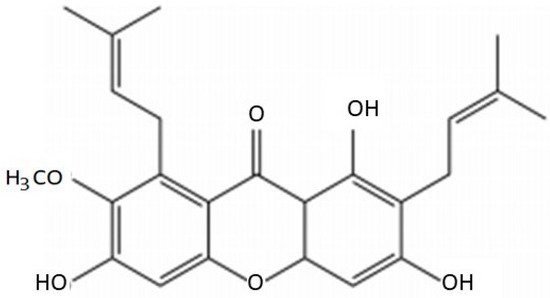
Figure 1. Composition of α-mangostin.
3. The Activity of AMG in Breast Cancer
Over 200 novel chemical compounds have been licensed for use in the battle against cancer in the last 50 years, with about 50% being molecules of unaltered natural products [
21]. Small organic molecules of secondary metabolites have played an essential role in inhibiting proliferation-induced apoptosis or other modifiable mechanisms [
22,
23,
24]. Natural products and their derivatives have been recognized for many years by the pharmaceutical industry [
25,
26].
3.1. Molecular Development of Breast Cancer
BC is one of the most well-known cancer types, having various pathological subtypes and clinical outcomes. BC may be caused by a mutation that results from the aging process and risk factors associated with a healthy lifestyle [
27]. The most promising strategy for cancer treatment is to disrupt the three phases of carcinogenesis—initiation, promotion, and progression—and alter the carcinogenesis signaling pathways [
4]. BC treatment may be quite successful, mainly when the illness is detected early and has a favorable prognosis (
Figure 2) [
28,
29].
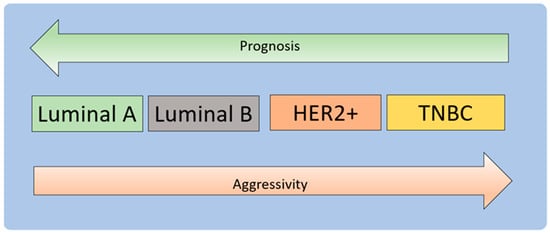
Figure 2. Molecular subtypes of breast carcinoma. BC can be based on hormonal receptors, and HER2 status is divided into luminal types A and B, HER2+, and TNBC [
30]. Luminal A and luminal B represent [ER+|PR+]HER2 (tumors with positive ER or PR and negative HER2) and [ER+|PR+]HER2+ subtypes (tumors with positive ER or PR and positive HER2). Luminal A tumors have higher expression of ER-associated genes and lower expression of proliferative genes than luminal B cancers [
31,
32]. Luminal B tumors tend to have a higher grade than luminal A tumors. The luminal subtype generally carries a good prognosis, and luminal B tumors have a much poorer prognosis than the luminal A subtype [
33]. The basal subtype comprises ER-PR-HER2- (triple-negative) tumors with an expression profile that mimics basal epithelial cells from other parts of the body and normal breast myoepithelial cells [
34]. This subtype has a low expression of hormone receptors and HER2 and high expression of basal markers and proliferation-associated genes. The tumor has a difficult prognosis, an aggressive clinical course, and currently lacks a standard targeted form of systemic therapy. The pattern of metastases tends to be visceral (excluding bone) and is less likely to involve lymph nodes. Tumors of this class tend to show rapid growth [
35].
Cancer develops through a series of molecular processes, namely mutations in DNA molecules that code for proteins that initiate cell division, proliferation, and growth. As a result, damage to DNA or proteins that regulate the cell cycle can result in uncontrolled cell division and proliferation, referred to as cancer. Cancer causes dysregulation of apoptosis, proliferation, angiogenesis, and metastasis [
4].
BC is molecularly classified into estrogen receptor (ER) and progesterone receptor (PR) expression and human epidermal growth factor receptor (HER2) amplification [
4]. Approximately 60–70% of early BC patients are hormone-sensitive, showing positive estrogen receptor (ER+), positive progesterone receptor (PR+), or both. About 15–20% of BC patients exhibit a triple-negative phenotype due to the absence of ER, PR, and HER2 amplification. Approximately 20% of BC cases show HER2 overexpression, resulting in aggressive disease and reduced survival [
36,
37].
BRCA1 is a human tumor-suppressor gene responsible for repairing damaged DNA or destroying cells if the DNA cannot be repaired. BC 1 (BCA1) and BC 2 (BCA2) are essential for genome stability. Mutations in genes give the risk of BC up to 15–20 times [
38,
39,
40].
The tumor suppressor TP53 gene encodes the protein p53, which regulates the cell cycle, induces apoptosis, maintains genome integrity, and prevents tumor development. Mutations in the TP53 gene increase the risk of BC [
4]. When stress occurs, phosphorylation of p53 interferes with binding to protein MDM2, resulting in p53 accumulation and subsequent transcription of several genes, including the gene encoding the protein cyclin-dependent kinase inhibitor (CKI) p21. P21 interacts with and inactivates the G1/S-Cdk complex, reprogramming cells to G1 for DNA repair [
41,
42].
BC treatment protects genomic stability by avoiding DNA damage, delaying the cell cycle or inducing apoptotic cell death, and influencing aberrant cell proliferation pathways [
4]. Chemotherapy can inactivate normal cell p53 and induce apoptosis in regularly developing cells/tissues, such as bone marrow, lymphoid organs, hair follicles, and small intestinal epithelium [
2,
43,
44].
3.2. Mechanisms of Molecules Drug Resistance (MDR)
Overexpression of MDR1 is one of the main reasons for multidrug resistance to chemotherapeutic drugs [
45]. The expression of P-glycoprotein encoded by the multidrug resistance (
MDR1) gene is associated with the emergence of the MDR phenotype in cancer cells [
46]. P-glycoprotein (P-gp) is a well-identified membrane transporter with the capability to efflux drug molecules out of the cancer cell, leading to the reduced efficiency of chemotherapy [
47]. MDR mechanisms fall into different categories: (1) enhance the efflux of drugs across membrane carriers, using ATP binding cassette (ABC) carriers as major carriers; (2) diminished uptake of drugs via influx carriers, such as solvent carriers; (3) enhanced metabolic drugs, including the removal of S-transferase glutathione and P450 cytochrome enzyme; and (4) inhibiting of apoptotic pathways [
48,
49,
50,
51].
3.3. Cytotoxicity
Cytotoxicity refers to a poisonous agent’s toxic effect on cells. Cytotoxic agents inhibit cell growth and can occasionally result in cell death [
52]. Exposure of cells to cytotoxic substances can result in various cell-death consequences [
53,
54,
55]. Following that, cells may initiate a program of programmed cell death (apoptosis) or necrosis [
56,
57]. Cells that undergo fast necrosis in vitro lack the time and energy necessary to activate apoptotic pathways and do not exhibit apoptotic markers. Secondary necrosis occurs when apoptotic cells in culture experience apoptosis [
58]. The causes of cytotoxicity are divided into the following: (1) Chemical cytotoxic agents—the primary goal for cytotoxic drugs, or cytostatic agents, is to prevent the growth of cancer cells, often accomplished by targeting processes that directly disrupt DNA replication or transcription interfering with critical cell processes during mitosis [
59]. (2) Biological cytotoxic agents—biological cytotoxic agents are commonly defined as harmful compounds produced from viruses, bacteria, fungus, plants, or animals. The most well-known compounds in this category are bacterial endo-/exotoxins and antibiotics [
58,
60]. (3) Physical cytotoxic agents—heat, ultrasonic vibrations, and radiation are all examples of physical agents that are cytotoxic. It has been discovered that using ultrasonic microbubbles enhances the cytotoxicity of chemotherapy medicines on tumor cells. Numerous research studies on radiation’s cytotoxicity may be found in the literature [
59].
3.4. Mechanisms of Cell Death
Cell death is a type of cellular suicide that is regulated by an intracellular program and acts as an innate method to maintain the balance of cell survival. In humans, cells undergo three stages of aging, namely (1) proliferation for a certain number of cycles; (2) loss of replicative ability, and then inactivity for a certain period of time; and (3) then they dying and death [
61]. Many stimuli cause cell death, and the mode of cell death will follow one of two general patterns. The first is necrosis; oncotic necrosis is most often the result of a severe metabolic disorder and is characterized by cellular swelling, leading to the rupture of the plasma membrane with the release of intracellular contents. The second pattern is apoptosis, a form of programmed cell death. Apoptosis causes regular resorption of individual cells initiated by a well-defined pathway involving the activation of proteases called caspases [
62].
Apoptosis is essential for maintaining tissue homeostasis in balance with cell proliferation and death. Unwanted, old, damaged, and altered cells of an organism must be removed by apoptosis [
63]. Apoptosis also protects cells in disease states or harmful chemicals, for example, in immunological reactions [
64]. Moreover, p53 is a cellular stress sensor and a major pathway activator. The extrinsic pathway begins outside the cell when it is determined in the extracellular environment that the cell must be killed [
65,
66]. Clearing damaged organelles is necessary for autophagy to support cell survival. Cell death and cell survival are represented by apoptosis. Induction of caspases or degradation of endogenous apoptosis inhibitors can be preceded or enhanced by autophagy [
67]
In contrast to necrotic cell death, which usually occurs due to adenosine triphosphate (ATP) depletion, apoptosis is an ATP-requiring process. Different organelles (plasma membrane, mitochondria, nucleus, endoplasmic reticulum, and lysosomes) elicit cell-death signals. In particular, mitochondrial permeabilization and dysfunction usually develop in necrosis and apoptosis. In some cases, apoptosis and necrosis share a signaling pathway, resulting in programmed necrosis called necroptosis. In this way, apoptosis and necrosis may represent extreme endpoints on the phenotypic continuum of lost cell viability [
68].
4. Nanotechnology of AMG in Breast Cancer
Bioactive secondary metabolites, such as flavonoids, terpenoids, alkaloids, tannins, and others, have been recognized as potential medical resources [
89,
90]. As shown in
Section 3 and
Section 4, AMG has high cytotoxicity. However, AMG has a problem in solubility, thus resulting in having poor target selectivity in the human body. Furthermore, effort is needed to increase the solubility, selectivity, and efficacy of α-mangostin as a new drug candidate in clinical therapy.
Nanotechnology is predicted to alleviate some of the drawbacks of current therapies [
91,
92]. Nanotechnology produces materials of various types at the nanoscale level. NPs are a broad class of materials that includes particulate matter, with one dimension less than 100 nm at least [
16]. The physical and chemical properties of nanosized materials differ substantially from the properties of the same material in bulk [
93,
94]
Nanotechnology has emerged as a great strategy to overcome the challenges of systemic toxicity, low solubility, poor bioavailability, high plasma protein binding, non-target drug accumulation, poor drug-receptor/tissue interactions, low penetration, and internalization into target tissues, as well as sub-therapeutic pharmacologic responses. Its core properties and surface functionalization facilitate the delivery of therapeutic drugs or molecules and enhance therapeutic efficacy [
95,
96,
97,
98].
NPs attract great attention as multifunctional nanocarriers in DDSs, by combining the specific targeting with the transport and release of a contrast agent [
99,
100]. Several chemotherapeutics have been encapsulated in NP delivery systems to increase antitumor efficacy, inhibiting metastases, and decrease the effective dose and side effects [
101]. Cancer drug-targeting strategies can be further advanced by developing DDSs, using stimuli-responsive nanomaterials, which can be performed based on endogenous and exogenous stimulant factors [
102], including passive or active drug targeting in tumor tissues and improved intracellular penetration [
96].
The high entrapment efficiency can be attributed to the hydrogen bonding interactions between xanthones and carrier molecules. The substantial increase in xanthone solubility, endocytotic uptake of cationic NPs, and intracellular xanthone delivery may provide a novel drug delivery system for treating and preventing gastrointestinal tract tumors, especially small-intestine and colon tumors [
103].
AMG NPs are used to increase solubility in water, provide a controlled release, and create targeted delivery systems. The forms of NPs for AMG are polymeric NPs, nano micelles, liposomes, solid lipid NPs, nanofibers, and nanoemulsions. Advances in nanotechnology have provided a new way of improving drug delivery systems (DDS) for hydrophobic drugs. The poly(ethylene glycol)–poly(e-caprolactone)–poly(ethylene glycol) triblock copolymer (PEG–PCL–PEG, PECE) is a type of biodegradable and amphiphilic polymer micellar that is formed by the self-assembly of two hydrophilic PEGs and hydrophobic PCL, resulting in a hydrophobic inner core and a hydrophilic outer shell [
104].
Wathoni et al. showed that nanomicelle modification increased the solubility of AMG by more than 10,000-fold. The formulation of NPs affects their biopharmaceutical, pharmacokinetic, and pharmacodynamic aspects. In addition, polymeric NPs provide targeted delivery and significantly enhance AMG biodistribution to specific organs [
105].
Miftahul et al. showed electrospun PVP nanofibers of mangosteen peel extract, increased antioxidant properties, and solubility of AMG due to increased surface area, using a highly water-soluble excipient, PVP, as the main base for NPs increases drug solubility mediated by hydrogen bonding interactions between excipients and water molecules [
106]. Wathoni et al. demonstrated the effect of enhancing properties and compounds of AMG by using the AMG-Chitosan/Carrageenan NP system. These NPs impact the physicochemical properties of AMG, improve the poor water solubility profile, and increase cytotoxicity [
107].
In some cases, nanocarriers are combined with a targeting mediator to obtain a drug delivery system targeted by NPs to a specific target. Pharm et al. showed that AMG-loaded crosslinked silk-fibroin-based NPs maintain AMG’s apoptotic effect while exhibiting more significant cytotoxicity than the free drug [
108]. Bonafe et al. demonstrated that AMG loaded in CD44 Thioaptamer-tagged NPs reduces the number of BC cancer cells [
7]. NPs that mediate passive or active target delivery are generally prepared with a high affinity for the target and low affinity for normal cells [
105].
NPs can passively accumulate in the cancer cells because of the enhanced permeability and retention (EPR) effect [
109,
110,
111]. Furthermore, the capability of surface modifying NPs allows for active targeted cells by combining monoclonal antibodies and tumor-specific ligands with the NP [
112]. With the advantages of NPs, the NP drug delivery system can be enhanced the cytotoxicity of free AMG. Various benefits of NPs have been described above, but their use in delivering AMG in BC therapy has not been widely studied.
Through cytotoxic stress, NPs can impair normal cellular function and are responsible for membrane damage. NPs can be used in the therapy of BC by increasing the following: (1) technologies to strengthen the solubility and stability of anticancer drugs; (2) passive and active targeting used by nano-based modalities to selectively target malignant tissues/cells; (3) distribution of various drugs to help decrease drug resistance; (4) nano-based vehicles with controlled-release techniques for medication; (5) usage of nanoformulation, which is dependent on stimulus responsiveness; (6) drug efflux pathways can be blocked by nano-based vehicles; and (7) delivery of different medications and, thus, aims to minimize drug resistance [
113].
Understanding the mechanism of nanotoxicity is critical to the design of safe NP-based systems. On the other hand, there is emerging evidence that the cytotoxic potential of NPs can be exploited in cancer treatment. NPs may have therapeutic value and modulate apoptosis, necrosis, necroptosis, and autophagy [
61]. Cancer nanomedicine can be designed to remove cancer, and nanomaterials can cause micro gaps in the endothelial walls of blood vessels. The possibility of endothelial leakage caused by nanomaterials must be considered while developing future nanomedicines, particularly those used to treat cancer [
114]. The delivery of AMG with can be achieved with a nanoparticle system, in accordance with improvements in pharmacokinetic properties, drug release, and drug targeting [
105].
Nanotechnology has succeeded in increasing bioavailability and distribution, maintaining the integrity of compounds, and increasing the biological activity of cancer drugs. AMG-NPs inhibited pancreatic cancer cell growth and CSC characteristics in vitro and tumor growth, development, and metastasis in mice. AMG NPs provide clinical significance in cancer and other diseases. Gold-encapsulated AMG/polyethyleneimine/cyclodextrin (AuNPs/PEI/CD) NPs inhibited prostate cancer cell viability of PC-3 and DU-145. Furthermore, the poly(ethylene glycol)-poly(l-lactide) (PEG-PLA)-encapsulated AMG NPs ameliorated the neuropathology of Alzheimer’s disease by increasing the expression of low-density lipoprotein (LDLR) receptors in microglia and liver cells and accelerating clearance of amyloid-beta. In another study, oral administration of mucoadhesive NPs loaded with
Garcinia mangostana extract eradicated
Helicobacter pylori infection in mice [
115].