AhR is a ligand-activated transcription factor that belongs to basic-helix-loop-helix (bHLH)/Per-ARNT-Sim (PAS) family, which is involved in the regulation of cell differentiation, proliferation, and cancer imitation. AhR plays an important role in various physiological pathways, including host defense, immunity, stem cell maintenance, cell differentiation, and xenobiotic metabolism. It was initially believed that AhR is activated only by a group of environmental pollutants, such as polycyclic aromatic hydrocarbons (PAHs).
- autism
- environmental pollutants
- AhR
- CYP1A1
- epigenetic modifications
- polymorphism
1. Introduction
Autism spectrum disorder (ASD) is a disease that affects the development, communication, and behavior of an individual. It usually evolves during early childhood and persists throughout the lifetime of the affected individual, causing social and behavioral impairments. These defects may impede individuals with their day-to-day lives and make them dependent on the people around them [1]. Earlier understanding of autism was limited to a more severe variety of the spectrum, which resulted in pronounced speech defects, learning disabilities, and below-average IQ. Consequently, high-functioning individuals with variants of autism were misdiagnosed or underdiagnosed [1]. ASD is now used as an umbrella term encapsulating a large variation in developmental and behavioral disorders, including autism disorder, Asperger’s disorder, childhood disintegrative disorder, and pervasive developmental disorder—not otherwise specified [2].
Prevalence of autism has risen in recent years, from 0.4% in the 1960s–1970s to approximately 1.9%, affecting approximately 1 in 54 children in 2016 [3]. Although part of the increase could be attributed to better awareness and early detection, diagnostic drift does not completely account for the astounding numbers [4]. Individuals with ASD have symptoms and signs that are characteristically different from each other. Nonetheless, the diagnosis of ASD is dependent on two core domains, the social communication deficit and repetitive–restrictive behavior [5]. According to the Diagnostic and Statistical Manual of Mental Disorders criteria for ASD (DSM-5), social communication deficits are characterized by the impairment of socioemotional reciprocity, non-verbal communicative behaviors, and poor ability to develop, maintain, and understand relationships [6]. Manifestations of the repetitive–restrictive behavior include stereotyped or repetitive motor movements, inflexible adherence to routines or ritualized patterns of verbal and non-verbal behavior, abnormally high restricted or fixated interests, and hyper-reactivity or hypo-reactivity to sensory input [7].
The etiology of ASD is complex with several neurobiological factors contributing to its development, including genetic, epigenetic, and environmental factors [8,9]. Genetic influences have been recognized as the most likely cause of autism, in which approximately 50% ASD cases are inherited [10]. De novo mutations in the parent gametes have been established as a cause of sporadic occurrence of autism, accounting for nearly 30% of all autism cases in males and 45% in females [11]. Epigenetic causes, such as DNA methylation, genomic imprinting, and histone modification also instigate ASD [12,13,14]. Prenatal factors that induce changes in inflammatory cytokines, such as diabetes, Rubella, cytomegalovirus infections, and persistent fever during pregnancy are risk factors leading to autism [15]. In this regard, it has been shown that gene expression and plasma levels of several proinflammatory cytokines, prostaglandins, chemokines, and inflammation/oxidative stress-related proteins are highly elevated and strongly correlated with clinical features of children with ASD when compared with age-matched control [16,17,18,19]. Alternatively, a link between low thyroid hormone levels during pregnancy and an increased risk for autistic traits has been reported [20].
Although some factors contributing to the development of autism have been identified and characterized, these traditionally recognized risk factors alone cannot explain the rapid increase of autism incidence worldwide. There is extensive, ongoing research taking place to examine the possibility of the involvement of other risk factors, particularly exposure to environmental pollutants. The U.S. Environmental Protection Agency has reported an increase in the role of environmental factors in ASD incidence and development more than what was previously thought. In the past decades, uncontrolled industrialization and development of several human activities resulted in the emission of pollutants, such as heavy metals, aromatic hydrocarbons, dioxins, and phthalates, into the air, water, and soil, finally resulting in a massive environmental pollution. Consequently, people have been exposed to a mixture of these environmental chemicals/pollutants unknowingly, continually, and chronically. Co-contamination with complex mixtures of environmental pollutants is a common environmental problem with multiple biological consequences, particularly to the enzyme systems and metabolism in the body. The American and Canadian agencies for Environmental Protection Act rank heavy metals, polycyclic aromatic hydrocarbons (PAHs), and other environmental pollutants among the most hazardous and toxic substances in the environment [21,22]. The increase in everyday exposure and accumulation of environmental toxins through air, water, soil, and food have been revealed to play a critical role in the pathogenesis of several diseases, such as cardiovascular diseases [23,24], cancer [25,26], respiratory diseases [26,27], diabetes mellitus [28], and neurological diseases [29].
2. Environmental Pollution and ASD
Carter and Blizard have conducted a study to examine ASD gene-environment interaction and showed that approximately 67,861 chemical–gene interactions affected the autism susceptibility genes (ASGs), among which 4428 environmental toxins and chemicals affected one or more ASG, suggesting that ASGs are targets for environmental toxins [34]. For example, autism has been linked with organochlorine insecticides via disruption of pre- and post-synaptic dopamine, GABA, and glutamate function [34]. Another example of a close relationship between genes and the environment is the toxicogenomic effect of copper metal on reducing ProSAP/Shank protein levels in the brain, and decreasing the expression of the N-methyl-D-Aspartate receptor (GRIN1) in the excitatory synapse associated with autism [35]. A recent nationwide case-control study conducted in Denmark, which included 15,387 children with autism and 68,139 healthy age- and sex-matched children, revealed that exposure to air pollution in early stages of infancy, but not during pregnancy, contributed to increased risk of ASD [33].
Particulate matters (PMs) are small air pollutants composed of microscopic solid particles or liquid droplets that can easily enter the lungs and cause diseases, including autism [36]. PMs are one of the most investigated air pollutants with regards to autism as they carry all hazardous particles suspended in the air, including organic compounds, diesel exhaust, polycyclic aromatic hydrocarbons, endotoxins, and reactive heavy metals [37]. Traffic, domestic heating, and industrial emission are major contributors to ambient PM concentrations [38]. For example, traffic diesel exhaust has been estimated to contribute to more than 35% of air PM [39] and, thus, increased autism incidence was positively correlated with proximity to highways and exposure to PM 10 during the third trimester, with an estimated odds ratio of 1.36 [32,40,41,42]. Although several studies have shown an association between PM and ASD; specific evidence for individual constituents is conflicting. A case-control study in Shanghai reported an increased risk of developing ASD among children below 3 years who were exposed to PMs [43]. Systematic reviews and meta-analysis studies conducted to review epidemiological literature on the impact of PM exposure on ASD showed a strong evidence of association for prenatal and postnatal exposure to PM 2.5 , with little evidence for PM 10 [44,45,46]. Geng and coworkers have demonstrated a significant correlation between autism severity and the PM 2.5 serum levels of children with autism [47]. At the experimental level, exposure of neonatal male Sprague–Dawley rats to PM 2.5 caused autism-like behavioral abnormalities, such as communication deficits, decreased social interaction, and aversion to unfamiliar objects [48]. This delirious effect could be possibly attributed to induction of neuroinflammation, dysregulation of immune system, mutation of a pivotal gene involved in formation, maturation, and maintenance of synapses [48], and DNA methylation and oxidative stress [49].
Heavy metals are stable air pollutants that are neither created nor biodegradable, making the exposure to metallic elements a matter of growing concern [50]. Heavy metals are known as neurodevelopmental toxicants causing fetal damage and neurological diseases, including autism. Several case-control studies revealed that chronic exposure to inorganic mercury resulted in a staggering 60% increase in susceptibility to autism. Recent systematic reviews and meta-analysis studies showed that ASD patients exhibited higher concentrations of heavy metals, such as antimony, mercury, lead, in their hair and blood, which were positively associated with an increased risk of autism [46,51]. In addition, the higher levels of heavy metals in children with autism than matched controls were positively correlated with maternal fish consumption, maternal use of dental amalgam, residing near gasoline stations, and usage of aluminum pans [52]. These results imply that development index of countries significantly influences the overall concentrations of heavy metal toxicity between patients with ASD and control subjects, which support the theory that environmental pollution is a contributory factor to ASD.
Organic pollutants are long-lived toxic substances in the environment. They are generally divided into persistent and non-persistent, where non-persistent organic pollutants (NPOPs) are toxins that do not remain in the human body, but still harmfully affect various physiological pathways. The link between exposure to NPOPs, such as phthalate and bisphenol A (BPA), and autism development in children, has been reported in several studies that showed higher levels of phthalates and BPA compounds in the blood and urine of children with autism compared to healthy subjects [53,54,55]. On the contrary, other studies demonstrated that prenatal exposure to phthalates during the second and third trimesters of pregnancy was not associated with an increased risk of autism in children from this cohort [56]. On the other hand, persistent organic pollutants (POPs), such as organochlorine pesticides, polychlorinated biphenyls (PCBs), perfluoroalkyl substances (PFAS), polychlorinated dibenzofurans (PCDFs), and polycyclic aromatic hydrocarbons (PAHs) are generated from anthropogenic activities that resulted in the accumulation of these toxic substances in the soil, air, and water [34,57]. The direct link between exposure to POPs and autism has been reported. A population-based case-control study aimed to determine the impact of prenatal exposure to PCBs during pregnancy on autism demonstrated that elevated levels of PCB138/158, 155, and 170, were associated with higher risk of development of ASD, probably through specific gene modulations [58]. In addition, exposure to PCBs causes 15q11-q13 duplication autism spectrum disorder and development of autistic traits [59,60,61,62]. Further supporting evidence for the association of environmental pollutants and autism is the observation that elevated levels of 2,3,7,8,-tetrachlorodibenzo-p-dioxin (TCDD), a well-known PAH, in breast milk, increased autistic traits of 3-year-old children in Vietnam [63]. Importantly, these POPs and PAHs are known to exhibit their toxic effects on the human body through the activation of a cytosolic protein known as the aryl hydrocarbon receptor (AhR) [21], suggesting high possibility that the AhR pathway could mediate increased autism development and incidence. The next part of the review discusses recent advances and studies, highlighting the impact and role of the AhR pathway in the incidence of autism.
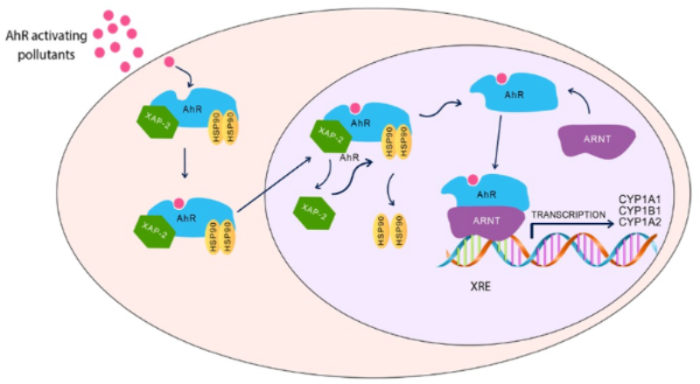
3. Aryl Hydrocarbon Receptor Pathway and ASD
Dioxin-like chemicals are well-known neurotoxic pollutants, where exposure to these chemicals has been linked with increased the risk of autism. Since these environmental toxicants target AhR to mediate their toxicities, it is highly possible that AhR could play a role in autism development during childhood; however, the links between AhR and autism are still not fully revealed. What supports this possibility is that AhR and its regulated genes, CYP1A1, CYP1A2, and CYP1B1, are highly and constitutively expressed in the placenta, which may be activated by exposure to environmental toxicants during pregnancy and, hence, increase the incidence of autism [78]. In addition, activation of the AhR/CYP1 results in DNA adduct formation and DNA strand breakage [79,80], considered as risk factors for the development of autism [80]. These results collectively indicate that AhR/CYP1 could play a role in ASD incidence. This section summarizes the most recent human and experimental studies ( Table 1 ) and evidence for the potential role of AhR and its regulated genes, CYP1A1, CYP1B1, and CYP1A2 on autism development.
| Gene | Study Model | Study Design | AhR/CYP1 Modulator | Effect on AhR/CYP1 Pathway | Effect on Autism Incidence & Development | References |
|---|---|---|---|---|---|---|
| AhR | Human | SK-N-SH human-derived neurons | TCDD | AhR activation → ↓AChE | ↓ Neuronal activity | [90] |
| CH223191 | AhR inhibitor, CH223191 → ↑ AChE | ↑ Neuronal activity | ||||
| Pregnant amniotic fluids | PCBs & heavy metals | ↑ AhR transactivation | ↓ The levels of PFAS were lower in ASD cases compared to control. | [78] | ||
| Rats | Perinatal exposure to TCDD | TCDD | Activation of AhR → ↓ AChE, monoamines, ↑ stimulation of GABA ↓ thyroid hormones, increase in TSH, decrease growth hormones in cerebellum of offspring |
↑ Permanent brain damage. Impaired the development of cerebellum of their offspring |
[89] | |
| CYP1A | Human | Autistic subject | ↓ CYP1A1 gene expression in umbilical cord blood | ↑ ASD incidence compared to control | [82] | |
| Pregnant | Dioxin | ↑ dioxin levels in maternal blood | ↑ Neurodevelopmental deficits and autistic traits in the children with ASD | [63,83] | ||
| PCDFs | ↑ PCDFs levels in maternal blood | ↑ Autistic traits in middle to late childhood using SRS | [61] | |||
| Zebrafish | Developmental exposure to PCB126 on early- and later-life behavioral | PCB126 | ↑ CYP1A1 in early stages of development, with no significant upregulation at adulthood | Impaired short-term and long-term habituation to unfamiliar environment ↑ Anxiety-related behavior with no change in the larval locomotor activity |
[91,92] | |
| Mice | Pregnant C57BL/6N | AhR plasmid transfection | Constitutive AhR activation | Affects neuronal migration during hippocampal development. | [84] | |
| High affinity Cyp1a2(−/−) | PCBs | CYP1A2 Knockout | ↑ Motor dysfunction compared to wild-type mouse. ↑ Susceptibility to nigrostriatal dysfunction and motor deficit, and toxicity of the cerebellum and cortex. |
[93,94] | ||
| CYP1B1 | Human | Autistic children | Vitamin D deficiency | ↓ CYP1B1 plasma levels by 70% through epigenetic silencing of CYP1B1 | ↓ Vitamin D by 60% → positively correlates with ASD | [81] |
Neuroinflammation has been hypothesized to contribute to autism development; for example, it was reported that the levels of pro-inflammatory cytokines are high in the blood and cerebrospinal fluid of patients with autism. A recent study on children with autism and age-matched healthy children showed elevated levels of AhR- mediated gene expressions of several inflammatory cytokines, such as interleukin-6 and signal transducer and activator of transcription 3 (STAT3) in children with autism, more than in healthy individuals [16]. This is supported by reports showing that STAT3 binds to its motif in the AhR promoter region; thus, activating AhR. There is a strong correlation between autism severity and the levels of vitamin D, in which children with autism are usually associated with vitamin D deficiency. Knowing that vitamin D is metabolized by CYP1B1, it is highly suggested that variation in CYP1B1 expression could play a role. The link between CYP1B1-mediated vitamin D deficiency and autism has been examined by El-Ansary and coworkers, who were the first to show that the plasma levels of CYP1B1 and vitamin D in 28 children with autism were 70% lower than their age- and sex-matched neurotypical children [81]. Although there is no other supporting study, it was postulated that decreased CYP1B1 levels in patients with ASD could be attributed to epigenetic silencing of the CYP1B1.
Meta-analysis studies across two prospective pregnancy cohorts showed that the CYP1A1 gene expression was downregulated in umbilical cord blood from subjects with autism, suggesting its involvement in ASD etiology [82]. In addition, it has been reported in two separate studies that exposure of Vietnamese and Taiwanese pregnant women to dioxin, an AhR activator, was associated with increased neurodevelopmental deficits and autistic traits in children with ASD [63,83]. A cohort study examined the impact of prenatal exposure to PCDFs on autistic traits in middle- to late childhood using the Social Responsiveness Scale (SRS), and found that higher levels of PCDFs in maternal blood during pregnancy were associated with lower SRS scores in children, which resulted in greater autistic-like social traits [61]. A recent case-control study showed that elevated levels of POPs (PCBs, dioxins, PFAS), elements, and heavy metals in the amniotic fluids of children with autistic traits were associated with increased transactivation of AhR [61]. This study provides evidence that environmental pollutants can cross the placenta and, hence, increase the risk of toxicities and ASD. Although the levels of PFAS were lower in ASD cases compared to the control, this could be explained by the possible removal of some PFAS congeners during the process of extraction, as PFASs are high albumin-binding compounds [78].
Autism-like behavior, including anxiety, locomotor activity, repetitive behavior, and altered swimming pattern was induced in zebrafish when gestationally exposed to heavy metal lead, which was associated with upregulation of CYP1A [91]. On the other hand, Glazer et al. have studied the impact of developmental exposure to low levels of PCB126 on early- and later-life behavioral phenotypes in the zebrafish model system [92]. In this study, adult behavioral assays, including shoaling and the novel tank assay, showed that exposure to PCB126 had impaired short- and long-term habituation to the unfamiliar environment, and exhibited high anxiety-related behavior with no change in the larval locomotor activity. These autistic effects of PCB126 were associated with a significant induction of CYP1A in early stages of development, with no significant upregulation at adulthood [92], implying that activation of AhR in the early developmental stages due to exposure to POPs is linked with autistic traits. In addition, Colter et al. and coworkers have used high affinity Ahr b Cyp1a2 (−/−) and poor affinity Ahr d Cyp1a2 (−/−) knockout mice models to study the effect of developmental PCB exposure on autism development [93]. In their studies, they found that both high- and poor-affinity knockout mice displayed motor dysfunction when exposed to high PCB levels during gestation and lactation, with higher susceptibility to nigrostriatal dysfunction and motor deficit in high-affinity Ahr b Cyp1a2 (−/−) knockout mice [93]. A follow-up study on the same mouse models has also established that high-affinity Ahr b Cyp1a2 (−/−) mice exposed to PCBs displayed the highest levels of toxicity and variation in gene expression in the cerebellum and cortex, the two centers of the brain responsible for motor activity and memory [94]. These studies clearly provide strong evidence for the involvement of the CYP1A2 gene in the neurotoxicity caused by developmental exposure to PCBs.
4. Molecular Mechanisms of AhR/CYP1A Regulation in ASD Development
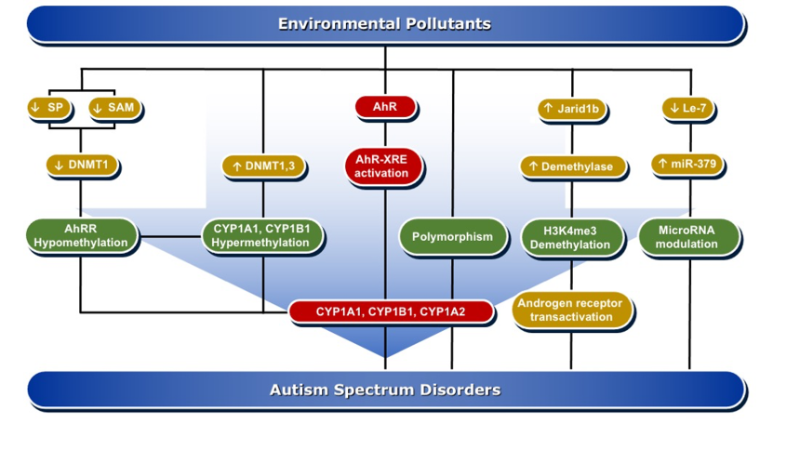
DNA methylation is one of the important mechanisms in epigenetics, in which cytosine is transformed into 5-methylcytosine by the transfer of a methyl group mediated by DNA methyltransferase (DNMTs) enzymes [98]. DNA methylation is pivotal in the regulation of gene expression, either by altering the recruitment of proteins or by hindering the binding of transcription factors to DNA. Both de novo hypermethylation and hypomethylation of the enhancer or promoter region of DNA during development are capable of changing the pattern of DNA methylation in the genome. This results in cell differentiation and development of a unique and stable DNA methylation pattern that can regulate tissue-specific gene transcription [99].
DNA methylation can affect brain tissue differentiation, nervous system development, and cause intellectual disorders, including autism. Mitchell et al. have reported that persistent exposure to organic pollutants, such as PCBs, causes epigenetic DNA methylation that is implicated in 15q11-q13 duplication autism spectrum disorder [59]. A genomic DNA study in postmortem individuals with ASD and in the cerebellum of BTBR T+tf/J autistic mice showed a significant increase in the expression levels of DNMT3a and DNMT3b as compared to non-autistic controls, which was positively correlated with the degree of DNA damage [100]. DNA methylation is one of the suggested mechanisms by which environmental pollutants, such as PCBs, are capable of inducing ASD development [101]. In that, the induction of oxidative DNA damage by AhR activation is aberrant DNA methylation [102]. An epigenome-wide study conducted in individuals perinatally exposed to PCBs and PCDFs compared to non-exposed subjects examined the methylation changes lasting to adulthood. The study showed differential DNA methylation for 20 CpGs mapped to 11 genes, including AhRR, CYP1B1, and CYP1A1 [102]. Men perinatally exposed to PCBs and PCDFs showed hypermethylation of CpG cg06264984 at CYP1B1, cg05549655 at CYP1A1, and cg17924476 in AHRR, with positive correlation with gestational levels of PCBs or PCDF toxic equivalency and exhibited hypomethylation of cg05575921 and cg21161138 in AhRR that were inversely related to PCB levels [102].
In experimental animal models, researchers have studied the effects of pre-and postnatal exposure to a mixture of AhR activators, such as PCBs, PCDD, methylmercury (MeHg), and organochlorine pesticides, on hepatic, uterus, and brain DNA methylation in prepubertal female Sprague–Dawley rats. In these studies, researchers found that the AhR activators induced CYP1A1 activity, which was associated with a significant decrease in the global genome DNA methylation and the mRNA levels for DNMT1, DNMT3a, and DNMT3b in brain homogenate and brain areas, such as hypothalamus, hippocampus, and cortex [107,108]. Mechanistically, inhibition of DNA methylation by the AhR activators is mediated through downregulation of Sp1, a regulator of DNMT1 expression in the brain [109] and the reduction of S-adenosyl methionine (SAM) concentrations, universal methyl donor involved in DNA methylation [107].
A harmonious communication between various hormones is imperative to proper neurodevelopment. Since ASD is more prevalent in males than females, endocrine disruption is hypothesized to be a contributory factor to ASD. It has been found that DNA methylation is a very important player in sex-specific gene expression. PCBs have influenced sexual differentiation [110]. In one study, it was found that exposure of Sprague–Dawley rats to a mixture of PCBs, Aroclor 1221 on gestational days 1, 3, 16, and 18, caused a delay in onset of puberty in males and estrous cyclicity in females [111]. These effects were associated with significant increases in the gene expression of DNMT1 and ARNT in a sex-specific manner. Increased DNA methylation in response to PCB exposure alters endocrine function and gene expression in the brain, which influence the sexual development and sex-specific transcriptional profile in the brain [101,111]. These studies support the possibility that the AhR/CYP1 pathway is involved in ASD development through DNA methylation changes, which could persist in the offspring for 20 years or even more, leading to diseases, including autism.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22179258
