The prevalence of chronic kidney disease (CKD) is increasing worldwide, and a close association between acute kidney injury (AKI) and CKD has recently been identified. Black cumin (Nigella sativa) has been shown to be effective in treating various kidney diseases. Accumulating evidence shows that black cumin and its vital compound, thymoquinone (TQ), can protect against kidney injury caused by various xenobiotics, namely chemotherapeutic agents, heavy metals, pesticides, and other environmental chemicals. Black cumin can also protect the kidneys from ischemic shock. The mechanisms underlying the kidney protective potential of black cumin and TQ include antioxidation, anti-inflammation, anti-apoptosis, and antifibrosis which are manifested in their regulatory role in the antioxidant defense system, NF-κB signaling, caspase pathways, and TGF-β signaling.
1. Introduction
Kidney diseases are considered as a global public health problem. Chronic kidney disease (CKD) is a critical regulator of morbidity and mortality from non-communicable diseases, while the incidence rate of acute kidney injury (AKI) is increasing worldwide [
1]. Patients with a history of AKI may develop CKD [
2,
3]. The pathophysiology of kidney disease is complex and includes inflammation, tubular injury, and vascular damage [
4,
5]. Being excretory organs, kidneys are particularly vulnerable to the toxic effects of xenobiotics and their metabolites. With the increasing exposure to xenobiotics such as drugs, toxins, and environmental chemicals, the global incidence of chronic human diseases including kidney disease is growing at an alarming rate [
6]. Xenobiotics impair the structural and functional capacity of kidneys by inducing oxidative stress, inflammation, apoptosis, and fibrosis, leading to the development of AKI and CKD [
6,
7]. Although the pathophysiology of various kidney diseases has been studied, many targeted clinical therapies have failed [
8]. Thus, urgent interventions are needed to treat patients with kidney disease.
Black cumin (
Nigella sativa L.) is a popular spicy herb and its seeds, in particular, have traditionally been indicated in the management of various human ailments, including those affecting the renal system [
9]. Thymoquinone (TQ), the main active component of black cumin seed and its oil, was shown to promote the function of different vital organs, including kidney function [
10]. Mounting evidence shows that black cumin and TQ can alleviate kidney complications caused by various stress factors, namely chemotherapeutic agents, metabolic deficits, and environmental toxicants [
11]. Evidence from the preclinical studies has shown that black cumin seed (in the form of powder, extracts, or oil) and TQ protect against kidney injuries induced by ischemia [
12,
13], cancer chemotherapeutic drugs (methotrexate and cisplatin) [
14,
15], analgesics (paracetamol, acetylsalicylic acid and aspirin) [
16,
17,
18], heavy metal (arsenic and cadmium) [
19,
20], pesticide (piconazole and diazinon) [
21,
22], and other chemicals (carbon tetrachloride and sodium nitrite) [
23,
24]. Evidence, athough limited, also suggests clinical improvements in CKD patients treated with black cumin [
25,
26,
27]. Besides, black cumin was shown to be effective in modifying various risk factors for kidney disease such as hypertension, atherosclerosis, dyslipidemia, hyperglycemia, and diabetes [
11]. The kidney-protective effects of black cumin are owing to its antioxidant, anti-inflammatory, immunomodulatory, antiapoptotic, and antifibrotic properties [
11,
28,
29].
2. Antioxidant and Anti-Inflammatory Effects of Black Cumin and TQ
Oxidative stress and inflammation are two pathogenic events that are known to be crucially implicated in the pathobiology of various kidney problems, including kidney toxicity, AKI, and CKD [
30,
31]. Many natural products have proven potential in alleviating oxidative stress and inflammation [
32,
33] and have thereby shown efficacy against kidney diseases (
Figure 1 and
Figure 2).
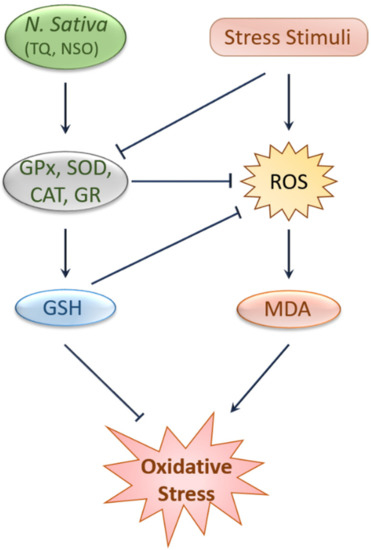
Substantial evidence from animal and human studies have confirmed the protective effects of black cumin and TQ against oxidative stress [
28,
34,
35,
36,
37,
38]. Black cumin upregulated erythrocyte glutathione peroxidase (GPx), glutathione-S-transferase (GST), and superoxide dismutase (SOD) levels and simultaneously lowered plasma malondialdehyde (MDA) levels [
38,
39]. In two similar studies, black cumin increased the level of antioxidant enzymes, such as SOD and catalase (CAT), and antioxidant molecules, such as glutathione (GSH) and decreased reactive oxygen species (ROS) [
40,
41]. Moreover,
N. sativa oil (NSO) reduced chlorpyrifos-induced oxidative stress by decreasing ROS and nitrous oxide production in the Wister rats model [
42]. Daily intake of TQ (5 mg/kg) for five weeks elevated CAT, glutathione reductase (GR), GPx, SOD, and GSH level in liver tissues [
43]. Similarly, TQ elevates SOD, CAT, and GSH levels, upregulates antioxidant genes, and downregulates pro-oxidant genes [
44]. Another study in rabbits revealed that consuming black cumin seeds (600 mg/kg) decreased MDA and increased total antioxidant levels in the blood [
45]. Again, combined supplementation of TQ and NSO exhibited antioxidant capabilities against cisplatin (CP)-induced abnormalities [
46]. One meta-analysis report on black cumin seed showed enhanced SOD levels without any visible effect on MDA level and total antioxidant capacity [
47]. Even so, this preclinical evidence of the antioxidant effects of black cumin has been elaborated in clinical studies. Combined ingestion of black cumin seed and
Allium sativum over eight weeks improved antioxidant status in 30 postmenopausal, healthy women [
39]. Again, supplementation of NSO and a low-calorie diet showed an improvement in antioxidant status in a clinical trial of 50 obese volunteers [
48].
Figure 1. Protection against oxidative stress by black cumin and its constituents. Stress stimuli like CP and chlorpyrifos reduce antioxidant enzymes and elevate ROS and MDA levels, leading to oxidative stress, which was attenuated by N. sativa and TQ through a mechanism involving the upregulation of antioxidants enzymes and molecules, such as GPx, GR, SOD, CAT, and GSH and the subsequent reduction of ROS and MDA levels. CAT, Catalase; GPx, Glutathione peroxidase; GSH, Glutathione; GR, Glutathione reductase; MDA, Malondialdehyde; NSO, N. sativa oil; ROS, Reactive oxygen species; SOD, Superoxide dismutase; TQ, Thymoquinone.
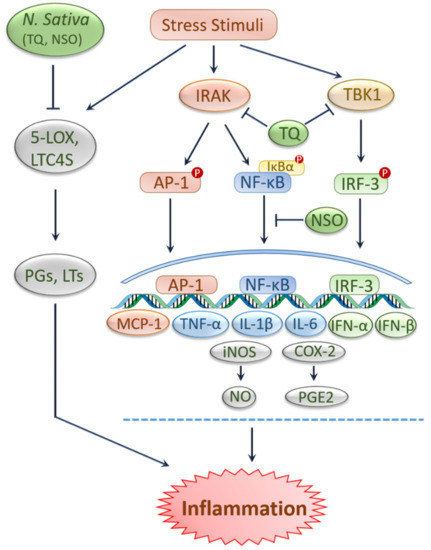
Along with protection against oxidative stress, black cumin and TQ have been shown to curb inflammation as claimed by previous literature [
9,
28,
35,
49]. The extracts and bioactive compounds of black cumin, such as TQ, nigellone, and α-hederin revealed anti-histaminic, anti-immunoglobulin, anti-leukotrienes, anti-eosinophilic, and anti-inflammatory effects in several models [
50]. In addition, TQ suppressed pro-inflammatory factors such as nitric oxide (NO), nitric oxide synthase (iNOS), tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and cyclooxygenase 2 (COX-2) by inhibiting IRAK-linked AP-1/NF-κB pathways [
51]. In human blood cells, NSO and TQ inhibited 5-lipoxygenase (5-LOX) and leukotriene C4 synthase (LTC4S) [
52], which may generate inflammatory mediators like leukotrienes and prostaglandins [
52,
53]. In another study, TQ inhibited TANK-binding kinase 1 (TBK1), lowered the type I interferons (IFN) mRNA expression and downregulated the interferon regulatory factor 3 (IRF-3) signaling pathways in lipopolysaccharides (LPS)-stimulated murine macrophage-like RAW264.7 cells [
54]. In lung tissue, NSO treatment caused a reduction in IgG1, IgG2a, interleukin-2 (IL-2), interleukin-12 (IL-12), interleukin-10 (IL-10), IFN-γ levels and inflammatory cells [
55]. Additionally, administration of NSO significantly reduced IL-6, slightly reduced IL-12, and TNF-α levels in rats affected with carrageenan-induced paw edema [
56]. Similarly, supplementation of 10% NSO alleviated inflammation in paw edema rats with a lessened leucocytes count and TNF-α level [
49]. Again, an experiment in human pre-adipocytes demonstrated that the fresh extracted and stored NSO resulted in decreased IL-6 and IL-1β levels, respectively [
57].
Figure 2. Protection against inflammation by black cumin and its constituents. Stimulation of various extrinsic and intrinsic stressors triggers inflammatory signals. Activity of inflammatory enzymes such as 5-LOX and LTC4S resulted in the generation of leukotrienes and prostaglandins, respectively, leading to inflammation. NSO and TQ prevent inflammation by inhibiting 5-LOX and LTC4S. NSO reduces inflammation by downregulating IL-6. TQ suppresses pro-inflammatory cytokines by inhibiting AP-1/NF-κB pathways. TQ inhibits TBK1 and lowers IFN expression by downregulating IRF-3. AP-1, Activated protein-1; 5-LOX, 5-lipoxygenase; IFN-α, Interferon alfa; IFN-β, Interferon beta; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; IRF-3, Interferon regulatory factor 3; IRAK, interleukin-1 receptor-associated kinase; LTC4S, leukotriene C4 synthase; LTs, leukotrienes; MCP-1, monocyte chemoattractant protein 1; NF-κB, Nuclear factor-kappa B; NO, nitric oxide; NSO, N. sativa oil; PGs, prostaglandins; PGE2, Prostaglandin E2, TBK1, TANK-binding kinase 1; TNF-α, Tumor necrosis factor-alpha; TQ, thymoquinone; COX-2, cyclooxygenase 2; iNOS, nitric oxide synthase.
3. Protective Effects of Black Cumin and TQ against Kidney Injury
Black cumin and TQ have been reported to alleviating various abnormalities that often interfere with the physiological function of kidneys. In the following sections, the kidney-protective effects of black cumin and TQ are discussed, highlighting the underlying pharmacological effects (Table 1 and Table 2).
Table 1. Summary on the protective effects of black cumin and TQ against various experimental kidney injury models.
|
Experimental Models
|
Treatment with Doses
|
Pathophysiological Alterations
|
Ref.
|
|
Acetylsalicylic acid-induced nephrotoxicity in rats
|
Ethanolic NSE (250 mg/kg)
|
Improved paired kidney weight, body weight, relative tissue body weight index, and normalized serum urea and creatinine
|
[18]
|
|
Aspirin-induced nephrotoxicity in rats
|
Ethanolic NSE (250 mg/kg)
|
Significant improvement in histological parameters, including disrupted brush border, epithelial necrosis, intraluminal protein casts, and basement membrane integrity
|
[17]
|
|
Calcium oxalate-induced urolithiasis in rats
|
NSO (5 mL/kg BW/dose/
day for 28 days)
|
↓Urinary and serum rates of calcium phosphate and oxalate;
↑volume of urine excreted
|
[71]
|
|
CCl4-induced kidney injury in rats
|
Combined fish oil/ NSO (300 mg oil emulsions /kg BW, for 20 days)
|
↑Unsaturated fatty acids; ↓oxidative stress and inflammation
|
[69]
|
|
CP-induced AKI in rats
|
NSO (2 mL/kg BW orally)
|
↓Serum creatinine, BUN and ↑BBM enzyme activities in kidney cortical and medullary homogenates and BBMV; carbohydrate metabolism enzyme activities, and in the enzymatic and non-enzymatic antioxidant parameters toward normalcy
|
[15]
|
|
CP-induced kidney toxicity in rats
|
NSP (3 g/kg/day), extract (0.5g/kg/ day) and NSO (2 g/kg/day) for 60 days
|
↓Serum levels of urea, creatinine, and K+; ↑Na+, Na+/K+ ratio, vitamin D, nutritional markers, and antioxidant enzymes
|
[60]
|
|
Diazinon-induced nephrotoxicity in rats
|
NSO (2 mg/kg/daily)
|
↓AST, ALT, ALP, BIL, creatinine and urea
|
[22]
|
|
Haloperidol (HAL)-induced nephrotoxicity in rats
|
NSO (Pre-, co- and post-treatment: 150 mg/kg BW for 7 days)
|
↓K+, Na+, MDA contents and aldose-reductase activity, and AMP hydrolysis; ↑ATP in the plasma cell membranes of rat kidney; ↓inner kidney cortex and outer medulla
|
[61]
|
|
IRI-induced kidney injury in rats
|
Single dose of NSP (400 mg/kg orally)
|
↓Stain-positive cells in kidney tissue; ↓tissue MDA levels; ↑GPx and CAT
|
[12]
|
|
Methotrexate-induced nephrotoxicity in mice
|
NSO (0.125 mL/daily)
|
↓MDA; ↑GSH levels in kidney homogenate
|
[14]
|
|
Paracetamol-induced nephrotoxicity in rats
|
Ethanolic NSE (250, 500 and 1000 mg/kg)
|
↓Serum urea and creatinine; ↑SOD and GSH; ↓MDA levels in the kidneys; reversed kidney pathological damage
|
[16]
|
|
Penconazole-induced nephrotoxicity in rats
|
NSO (orally 0.2ml black cumin oil /100 g BW three days/ week for four weeks)
|
↓Subcapsular space and hypercellularity of the glomerular cells; attachment of podocytes and their processes; ↑Bcl-2 immune marker; ↓intercalated cells of cortical; ↓α-SMA and collagen fibers; ↓MDA level; ↑SOD and CAT
|
[21]
|
|
Sodium nitrite-induced nephrotoxicity in rats
|
NSO (2.5, 5, and 10 mL/kg for 12 weeks)
|
↓Serum urea and creatinine;
↑normal appearance of kidney tissue;
↓glycogen levels; ↓fibrosis markers, partially; ↓caspase-3 and pJNK/JNK
|
[70]
|
|
Unilateral ureteral obstruction-induced kidney damage in rats
|
NSE (200 and 400 mg/kg, 2 doses for 18 days)
|
↓Kidney angiotensin II and monocyte chemoattractant protein-1 expression, MDA and TNF-α levels, and the number of apoptotic cells; ↑kidney total thiol content and the activity of antioxidant enzymes
|
[72]
|
|
Arsenic-induced kidney toxicity in female rats
|
TQ (10 mg/kg) and ebselen (5 mg/kg)
|
↓Oxidative stress, inflammation, apoptosis, As accumulation in the kidney tissue;
↓histological kidney damage
|
[19]
|
|
Cadmium-induced nephrotoxicity in rats
|
TQ (50 mg/kg BW)
|
↓Toxicity of Cd and preserved histological architecture of the kidney tissue;
↓Overexpression of NF-κB in kidney tissue; ↓apoptotic cells; subdued lipid peroxidation; ↓SOD, GPx, and CAT activities in kidney tissue
|
[20]
|
|
IRI-induced kidney injury in rats
|
TQ (10 mg/kg/day)
|
Reduction of IRI-related alteration in kidney functions: ↑left RBF and GFR; ↑left kidney FENa; ↓gene expressions of KIM-1, NGAL, TNF-α, TGF-β1 and PAI-1
|
[13]
|
|
Sodium nitrite-induced kidney toxicity in rats
|
TQ (25 and 50 mg/kg, p.o., daily)
|
↓Oxidative stress, restoration of pro- and anti-inflammatory cytokines and protection of kidney tissue from apoptosis
|
[24]
|
|
CP-induced nephrotoxicity in rats
|
NSO (2 mL/kg BW, orally) and TQ (1.5 mg/kg BW, orally)
|
Improve kidney function, restored serum creatinine and blood urea nitrogen levels; ↑BBM marker enzymes (ALP, GGTase and LAP) in BBMVs, homogenates of kidney cortex and medulla; ↓kidney metabolic and redox status
|
[59]
|
AKI, Acute kidney injury; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AMP, Activated protein kinase; AST, Aspartate aminotransferase; ATP, Adenosine triphosphate, As, Arsenic; BBM, Brush border membrane; BBMV, Brush border membrane vesicle; BIL, Bilirubin; BUN, Blood urea nitrogen; Bcl-2, B-cell lymphoma 2; CAT, Catalase; CCl4, Carbon tetrachloride; CKD, Chronic kidney disease; CP, Cisplatin; Cd, Cadmium; FENa, Fractional excretion of sodium; GFR, Growth factor receptor; GGTase, Geranylgeranyltransferase; GPx, Glutathione peroxidase; GSH, Glutathione; IRI–Ischemia-reperfusion injury; JNK, c-Jun N-terminal kinases; KIM-1, Kidney injury molecule-1; LAP, latency-associated peptide; MDA, Malondialdehyde; NF-κB, Nuclear factor kappa B; NGAL, Neutrophil gelatinase-associated lipocalin; NSO, N. sativa oil; NSP, N. sativa seed powder; NSE, N. sativa seed extract; pJNK, Phosphorylated c-Jun N-terminal kinase; PAI-1, plasminogen activator inhibitor-1; RBF, Renal blood flow; SOD, Superoxide dismutase; TGF-β1, Transforming growth factor beta 1; TNF-α, Tumor necrosis factor alpha; TQ, Thymoquinone; α-SMA, Smooth muscle alpha-actin.
Table 2. Summary on the protective effects of black cumin against various kidney diseases in patients.
|
Types of Kidney Disease
|
Treatment with Doses
|
Pathophysiological Alterations
|
Ref.
|
|
Randomized, prospective, comparative, and open-labeled clinical trial with Stages 3 and 4 CKD patients
|
NSO (2.5 mL, p.o., once daily) along with alpha-keto analog of essential amino acids
|
↓Blood urea, serum creatinine, and 24-h total urine protein; ↑24-h total urine volume and glomerular filtration rate; delaying the progression of CKD at stages 3 and 4
|
[27]
|
|
Prospective, comparative, and open-label study with patients with CKD (Stage 3 and 4) due to diabetic nephropathy
|
NSO (2.5 mL, once daily and orally)
|
↓Blood glucose, serum creatinine, blood urea, 24 h total urinary protein levels;
↑glomerular filtration rate, 24 h total urinary volume, and hemoglobin level
|
[25]
|
|
Randomized, triple-blind, placebo-controlled, clinical trial in patients with kidney stones
|
Seed capsule (500 mg, twice for 10 weeks
|
Retreated or decreased the size of kidney stones
|
[26]
|
CKD, chronic kidney disease; NSO, Nigella sativa oil.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22169078