The muskrat is a neozoon species that has occupied many countries of continental North Europe after its introduction from north America as fur animals. Due to its burrowing activity it damages river and canal banks and structures of flood control. For this reason, the eradication of this alien species is recommended. Muskrats are also of parasitological interest since they can act as suitable intermediate hosts for Echinococcus multilocularis. On the other hand, little is known on the other helminths that infect muskrats. A total of 130 muskrats of different age groups trapped in different habitats in the Barnim district of the Brandenburg state by a professional hunter were examined for parasites and seven trematodes (Echinostoma sp., Notocotylus noyeri, Plagiorchis elegans, Plagiorchis arvicolae, Psilosostoma simillimum, P. spiculigerum, Opisthorchis felineus and four larval cestode species (Hydatigera taeniaeformis, Taenia martis, Taenia polyacantha, Taenia crassiceps) were detected. Larval stages of E. multilocularis were not found. O. felineus was found for the first time in muskrats in Germany. All the named parasites were present in Europe prior to the introduction of muskrats. With a prevalence of 48.9%, Strobilocercus fasciolaris, the larval stage of the cat tapeworm, H. taeniaeformis, was the most frequent parasite found in adult muskrats.
- Ondatra zibethicus
- helminths
- Federal State of Brandenburg
- Germany
1. Introduction
2. Analysis on Results
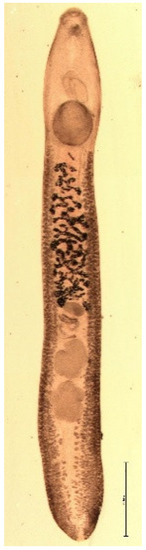
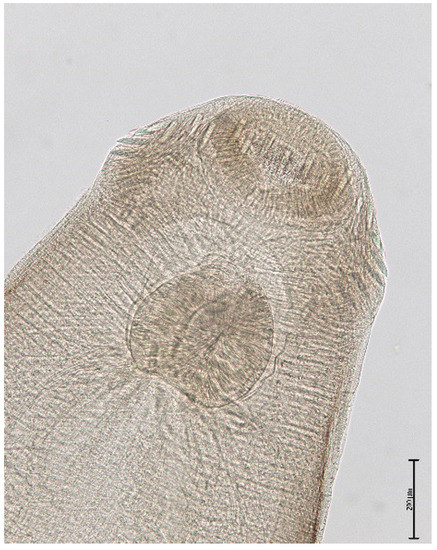
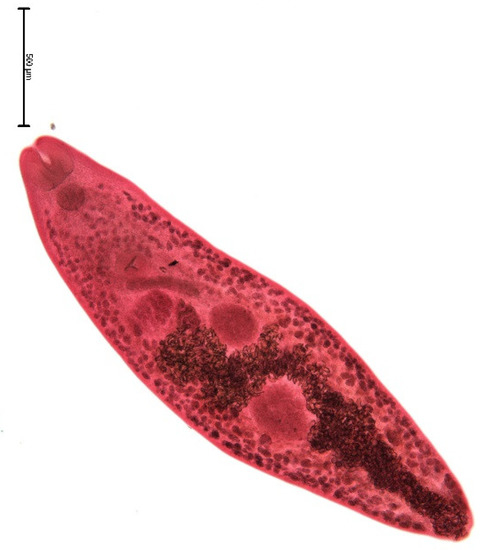
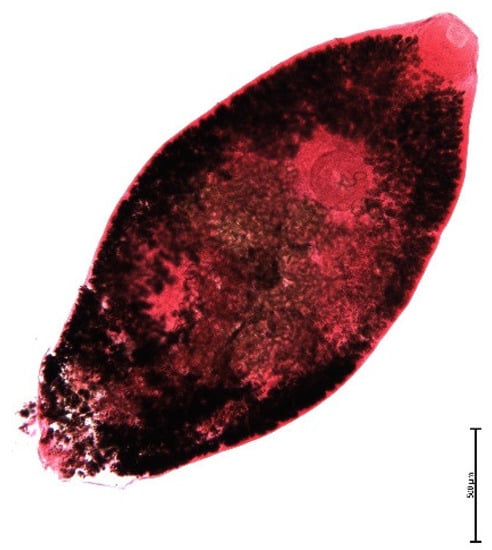
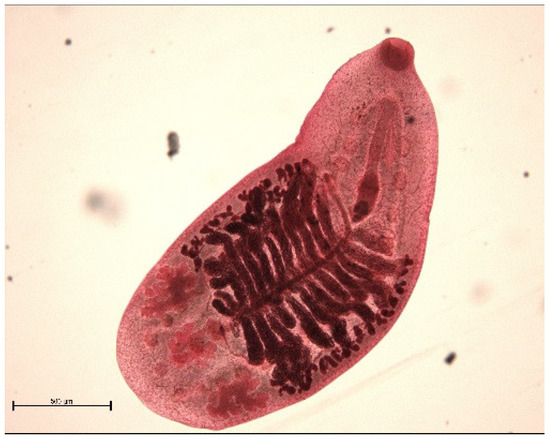
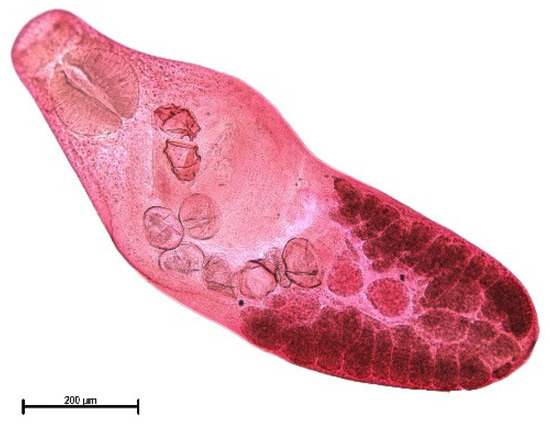
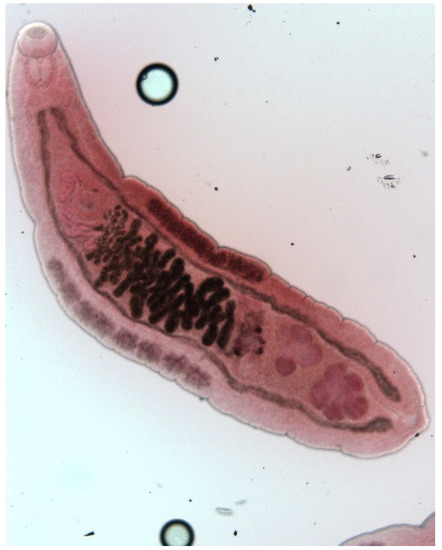

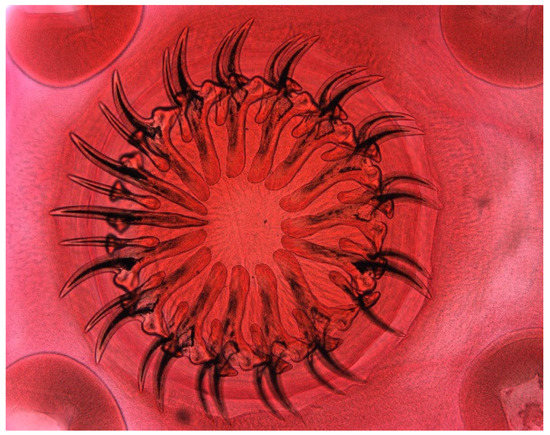
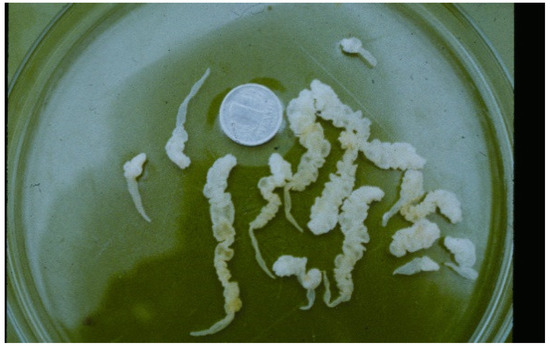
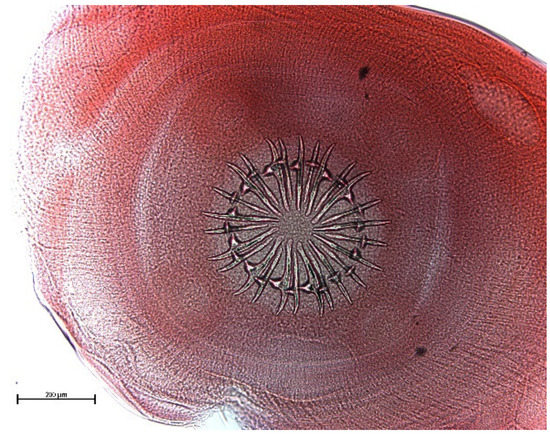
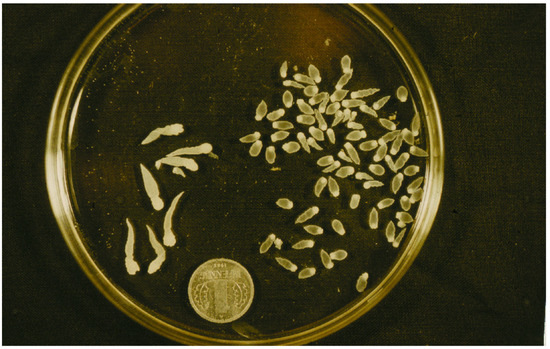
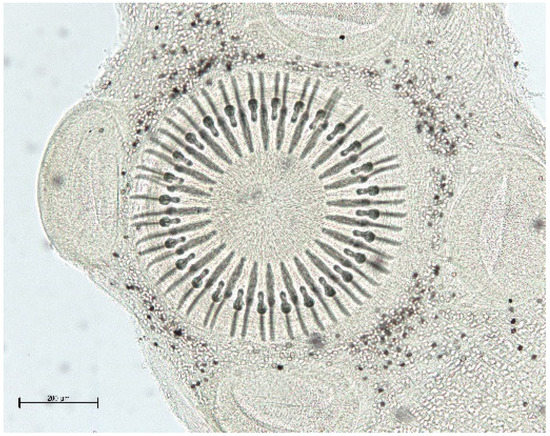
| Habitat | Number of Muskrats Infected with Parasites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Echinostoma sp. |
Plagiorchis elegans |
Plagiorchis arvicola |
Notocotylus noyeri |
Psilotrema spp. |
Opisthorchis felineus |
Hydatigera taeniaeformis |
Taenia martis | Taenia polyacantha |
Taenia crassiceps | |
| Lakes (n = 57) |
2 | 13 | 0 | 1 | 14 | 0 | 19 | 3 | 3 | 1 |
| Cut off meanders (n = 39) | 3 | 7 | 0 | 0 | 18 | 1 | 5 | 5 | 1 | 0 |
| Running waters (n = 34) | 7 | 1 | 1 | 0 | 1 | 0 | 6 | 0 | 0 | 0 |
| Total (n = 130) |
12 | 21 | 1 | 1 | 33 | 1 | 30 | 8 | 4 | 1 |
3. Current Insights
| Age Group |
Parasites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Echinostoma sp. |
Plagiorchis elegans |
Plagiorchis arvicola |
Notocotylus noyeri |
Psilotrema spp. |
Opisthorchis felineus |
Hydatigera taeniaeformis |
Taenia martis | Taenia polyacantha |
Taenia crassiceps | |
| Adults (n = 47) |
8.5 (5–62) |
17.0 (1–10) |
2.1 (5) |
0.0 (0) |
17.0 (1–15) |
2.1 (12) |
48.9 (1–15) |
10.6 (1–17) |
6.4 (1–69) |
2.1 (37) |
| Subadults (n = 31) |
6.5 (1–5) |
25.8 (1–8) |
0.0 (0) |
0.0 (0) |
35.5 (1–35) |
0.0 (0) |
12.9 (1–3) |
6.5 (1) |
3.2 (5) |
0.0 (0) |
| Juveniles (n = 52) |
11.5 (1–20) |
9.6 (1–2) |
0.0 (0) |
1.9 (3) |
25.0 (1–18) |
0.0 (0) |
3.8 (1–2) |
1.9 (1) |
0.0 (0) |
0.0 (0) |
| total (n = 130) |
9.2 (1–62) |
16.2 (1–10) |
0.8 (5) |
0.8 (3) |
24.6 (1–35) |
0.8 (12) |
23.1 (1–15) |
6.2 (1–17) |
3.1 (1–10) |
0.8 (37) |
| Cestode Larval Stage | [10] | [15] | [30] | [11] | [18] | [25] | [24] | This Paper |
|---|---|---|---|---|---|---|---|---|
| n = 630 | n = 437 | n = 670 | n = 80 | n = 991 | n = 1726 | n = 657 | n = 130 | |
| Germany | Germany | Germany | Germany | Germany | Netherlands | Belgium | Germany | |
| Echinococcus multilocularis | 0 | 1.8 | 0 | 0 | 4.1 | 0.1 | 22.1 | 0 |
| Hydatigera taeniaeformis | 33.17 | 48.1 | 99.6 | 63.75 | 42.3 | 44.8 | 65.8 | 23.1 |
| Taenia martis | 3.02 | 48.3 | 0 | 18.75 | 3.4 | 6.1 | 22.2 | 10.6 |
| Taenia crassiceps | 0.48 | 0.9 | 1.1 | 6.25 | 2.2 | 0.3 | 0.9 | 0 |
| Taenia polyacantha | 0.32 | 7.3 | 0 | 1.25 | 0.4 | 0.2 | 2.6 | 6.4 |
| Taenia pisiformis | 2.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Taenia mustellae | 4.8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mesocestoides sp. | 3.79 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
This entry is adapted from the peer-reviewed paper 10.3390/ani11082444
References
- Skyriene, G.; Paulauskas, A. Distribution of invasive muskrats (Ondatra zibethicus) and impact on ecosystems. Ekologija 2012, 58, 357–367.
- Ulbrich, J. The Muskrat: Biology, Distribution in Europe, Economic Significance and Control; C. Heinrich: Dresden, Germany, 1930; 137p.
- Serkowa, O.P. Parasite fauna of the muskrat acclimatized in the Kalelo-Finn SSR. Parasitol. Sbornik Zool. Inst. Akad. Nauk SSSR 1948, 10, 189–192. (In Russian)
- Dobrowolski, A.W. Parasites of the muskrat. Zool. J. 1952, 31, 640–642. (In Russian)
- DAISI. European Invasive Species Gateway. 2011. Available online: http://europe-alians.org/ (accessed on 16 May 2021).
- Bern Convention on the Conservation of European Wildlife and Habitats. Recommendation No 77(1999) on the Eradication of Non-Native Vertebrates. Available online: http://rm.coe.int/09000016807466df (accessed on 16 May 2021).
- Tackmann, K.; Löschner, U.; Mix, H.; Staubach, C.; Thulke, H.-H.; Conraths, F.-J. Spatial distribution patterns of Echinococcus multilocularis (Leuckart 1863) (Cestoda: Cyclophyllidea: Taeniidae) among red foxes in an endemic focus in Brandenburg, Germany. Epidemiol. Infect. 1998, 120, 101–109.
- Abuladze, K.I. Taeniata—Cestods of animals and man and diseases caused by them. In Essentials of Cestodology; Skrabin, K.I., Ed.; Nauka: Moskva, Russia, 1964; p. 530. (In Russian)
- Ganoe, L.S.; Brown, J.D.; Yabsley, M.J.; Lovallo, M.J.; Walter, W.D. A review of pathogens, diseases, and contaminants of muskrats (Ondatra zibethicus) in North America. Front. Vet. Sci. 2020, 7, 233.
- Müller, G. The muskrat in Saxony-Anhalt as intermediate host for cestodes. Zool. Garten Leipzig. N.F. 1952, 19, 42–44. (In German)
- Schuster, R. Contributions to the parasite fauna of the GDR. 8th communication: On the helminth fauna of Ondatra zibethica. Angew. Parasitol. 1987, 28, 21–25. (In German)
- Thiess, A.; Schuster, R.; Nöckler, K.; Mix, H. Helminth findings inindigenous raccoon dogs, Nycteroides procyonoides (Gray, 1834). Berl. Münch. Tierärztl. Wschr. 2001, 114, 273–276. (In German)
- Schwarz, S.; Sutor, A.; Staubach, C.; Mattis, R.; Tackmann, K.; Conraths, F.J. Estimated prevalence of Echinococcus multilocularis in raccoon dogs Nyctereutes procyonoides in northern Brandenburg, Germany. Curr. Zool. 2011, 57, 655–661.
- Schuster, R.K.; Shimalov, V.V. A comparative study of helminths of raccoon dogs (Nyctereutes procynoides) and red foxes (Vulpes vulpes) sharing the same territory. Asian Pac. J. Trop. Dis. 2017, 7, 705–711.
- Frank, B.; Zeyhle, E. Echinococcus and other tapeworm larvae in muskrat (Ondatra zibethicus). Nachrichtenbl. Dtsch. Pflanzenschutzd. 1981, 33, 166–170. (In German)
- Zeyhle, E.; Abel, M.; Frank, W. Epidemiological invstigations on the occurrence of Echinococcus multilocularis in final and intermediate hosts in the Federal Republic of Germany. Mitt. Österr. Ges. Tropenmed. Parasitol. 1990, 12, 221–232. (In German)
- Ewald, D. Distribution of the tapeworm Echinococcus multilocularis in the fox Vulpes vulpes and muskrat Ondatra zibethicus in the Freiburg administrative district. Mitt. Bad. Landesver. Naturkd. Naturschutz. 1990, 15, 81–100. (In German)
- Baumeister, S.; Pohlmeyer, K.; Kuschfeldt, S.; Stoye, M. On the prevalence of Echinococcus multilocularis and other metacestodes and cestodes of the muskrat (Ondrata zibethicus LINK 1795) in Niedersachsen. Dtsch. tierärztl. Wschr. 1997, 104, 448–452. (In German)
- Hartel, K.S.; Spittler, H.; Doering, H.; Winkelmann, J.; Hoerauf, A.; Reiter-Owona, I. The function of wild nutria (Myocastor coypus) as intermediate hosts for Echinococcus multilocularis in comparison to wild muskrats (Ondatra zibethicus). Int. J. Med. Microbiol. 2004, 293, 62–63.
- Schichowski, H.D. Investigations on the occurrence of finned stadia of Echinococcus multilocularis in muskrats (Ondatra zibethicus) in the district of Arnsberg North-Rhine). Z. Jagdwissenschaft 2002, 48, 119–124. (In German)
- Boussinesq, M.; Bresson, S.; Liance, M.; Houin, R. A new natural intermediate host of Echinococcus multilocularis in France: The muskrat (Ondatra zibethicus L.). Ann. Parasitol. Hum. Comp. 1986, 61, 431–434. (In French)
- Umhang, G.; Richomme, C.; Boucher, J.M.; Guedon, G.; Boué, F. Nutrias and muskrats as bioindicators for the presence of Echinococcus multilocularis in new endemic areas. Vet. Parasitol. 2013, 197, 283–287.
- Hanosset, R.; Saegerman, C.; Adant, S.; Massart, L.; Losson, B. Echinococcus multilocularis in Belgium: Prevalence in red foxes (Vulpes vulpes) and in different species of potential intermediate hosts. Vet. Parasitol. 2008, 151, 212–217.
- Mathy, A.; Hanosset, R.; Adant, S.; Losson, B. The carriage of larval Echinococcus multilocularis and other cestodes by the musk rat (Ondatra zibethicus) along the Ourthe river and its tributaries (Belgium). J. Wildl. Dis. 2009, 45, 279–287.
- Borgsteede, F.H.M.; Tibben, J.H.; van der Giessen, J.W.B. The musk rat (Ondatra zibethicus) as intermediate host of cestodes in the Netherlands. Vet. Parasitol. 2003, 117, 29–36.
- Sprehn, C. On diseases of German fur animals based on own research in 1929. Mitt. Reichszentr. Pelzt. Rauchw. Leipzig. 1930, 2, 61–69. (In German)
- Gässlein, H. Cestodes of vertebrates in the surroundings of Erlangen. Z. Parasitenk. 1954, 16, 443–468. (In German)
- Eble, H. Infection of muskrats with Cysticercus fasciolaris in the territory of the GDR. Wiss. Z. Univ. Halle 1957, 4, 159–166. (In German)
- Schuster, R. Cysticercus fasciolaris in the liver of a muskrat (Ondatra zibethica). Angew. Parasitol. 1982, 23, 223–227. (In German)
- Friedland, T.; Steiner, B.; Boeckeler, W. Prevalence of cysticercosis in muskrats Ondatra zibethica in Schleswig-Holstein west Germany. Z. Jagdwissenschaft 1985, 31, 134–139. (In German)
- Schuster, R.; Kaufmann, A.; Hering, S. Investigations on the endoparasite fauna of domestic cats in eastern Brandenburg. Berl. Münch. Tierärztl. Wschr. 1997, 110, 48–50. (In German)
- Schmidt, S. Examination of Wild Mice in Germany for the Occurrence of Capillaria hepatica and Cyclophyllidea. Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 2001.
- Kozlov, D.P. Key to the Helminths of Carnivorous Mammals of the USSR; Nauka: Moskva, Russia, 1977; 274p.
- Schuster, R.; Heidecke, D. Helminth findings in Castor fiber albicus MATSCHIE, 1907. Semiaquatische Säugetiere Wiss. Beitr. Univ. Halle 1992, 207–213. (In German)
- Schierhorn, K.; Stubbe, M.; Schuster, R.; Heidecke, D. Helminth fauna of the otter (Lutra lutra L., 1758). In Habitat 6; Reuter, C., Röchert, R., Eds.; GN—Gruppe Naturschutz.: Hamkensbüttel, Germany, 1991; pp. 133–142.
- Schuster, R.; Benitz, R. On the development of Taenia martis (Zeder, 1803) in the intermediate host. Helminthologia 1992, 29, 13–18.
- Kinsella, J.M. Growth, development, and intraspecific variation of Quinqueserialis quinqueseruialis (Trematoda: Notocotylidae) in rodent hosts. J. Parasitol. 1971, 57, 62–70.
- Seegers, G.; Baumeister, S.; Kuschfeldt, S.; Pohlmeyer, K.; Stoye, M. Nematode and trematode fauna of the muskrat (Ondatra zibethicus LINK, 1795) in Lower Saxony. Dtsch. Tierärztl. Wochenschr. 1997, 104, 503–504. (In German)
- Schuster, R. Echinostoma echinatum, Notocotylus noyeri and Quinqueserialis quinqueserialis as rare parasites of Rattus norvegicus. Angew. Parasitol. 1986, 27, 221–225. (In German)
