The Vitaceae Juss., in the basal lineages of Rosids, contains sixteen genera and 950 species, mainly of tropical lianas. The family has been divided in five tribes: Ampelopsideae, Cisseae, Cayratieae, Parthenocisseae and Viteae. Seed shape is variable in this family and is described based on the comparison of bi-dimensional seed images with geometric models.
Ten morphological types are described in the Vitaceae. Seven of them are general and three specific. Among the general types, three are shared with the Arecaceae and correspond to geometric figures well described (lenses, superellipses and elongated waterdrops). Four additional groups include waterdrops, normal or rounded, heart curves, normal or rounded, elongated heart curves and other elongated curves, respectively. Finally, the three specific types correspond to heart curves of the Cayratia and Pseudocayratia types, heart curves of the Squared Heart Curve (SqHC) type of Ampelocissus and Ampelopsis, and Elongated Superellipse-Heart Curves (ESHCs), frequent in Tetrastigma species and observed also in Cissus species and R. rhomboidea. All these groups are defined by geometric models obtained by the representation of algebraic equations. Modifications in the equations result in models adjusting to the shape of seeds for each species.
- endosperm
- geometry
- morphology
- seed shape
- Vitaceae
1. Introduction
| Tribe | Genera |
| I. Ampelopsideae (47) | Ampelopsis Michx. (18)Nekemias Raf. (9) |
| Rhoicissus Planch. (14) | |
| Clematicissus Planch. (6) | |
| II. Cisseae (300) | Cissus L. (300) |
| III. Cayratieae (368) | Cayratia Juss. (25) |
| Causonis Raf. (30) | |
| Acareosperma Gagnep. (1) | |
| Afrocayratia (7) | |
| Cyphostemma (Planch.) Alston (200) | |
| Pseudocayratia J. Wen,L.M.Lu and Z.D. Chen (5) | |
| Tetrastigma (Miq.) Planch. (100) | |
| IV. Parthenocisseae (16) | Parthenocissus Planch. (14) |
| Yua C.L.Li (2) | |
| V. Viteae (190) | Ampelocissus Planch. (115) |
| Vitis L. (75) |
2. Seed Morphology in the Vitaceae
2.1. Quantification of Seed Shape by Geometric Models
2.2. Seed Morphology in the Vitaceae
|
Tribe (Species Observed/Total) |
Genera (Species Observed/Total) |
Species (References for the Images) |
|
I. Ampelopsideae (15/47) |
Ampelopsis Michx. (13/18) |
Ampelopsis aconitifolia [36], A. arborea [37], A. bodinieri [36], A. cantoniensis [31,36], A. cordata [38], A. chaffanjoni [36], A. delavayana [31], A. denudata [30], A. glandulosa [39], A. grossedentata [31], A. humulifolia [36], A. japonica [36], A. megalophylla [31,36] |
|
|
Rhoicissus Planch. (2/14) |
Rhoicissus revoilii [31], R. rhomboidea [31] |
|
II. Cisseae (33/300) |
Cissus L. (33/300) |
Cissus antarctica [31], C. aralioides [35,40], C. barbeyana [40], C. bosseri [40], C. cactiformis [40], C. campestris [31,41,42], C. cornifolia [40], C. descoingsii, [31,41], C. diffusiflora [40], C. elongata [40], C. erosa [43], C. floribunda [40], C. fuliginea [31], C. granulosa [31], C. hastata [40], C. hypoglauca [31], C. integrifolia [40,42], C. leucophlea [40], C. penninervis [31], C. petiolata [40], C. pileata [40], C. populnea [40], C. quadrangularis [44], C. reniformis [31,41], C. repens [40], C. sciaphila [40], C. smithiana [40], C. sterculiifolia [31], C. subtetragona [40], C. trianae [31], C. tuberosa [42], C. verticillata [31,41,42,45], C. willardii [42], |
|
III. Cayratieae (40/365) |
Causonis Raf. (1/9) |
Causonis sp. [46] |
|
|
Cayratia Juss. (7/60) |
Cayratia cheniana [46], C. geniculata [31], C. imerinensis [47], C. japonica [31,48], C. oligocarpa [31], C. saponaria [31], C. sp. [African, 46] |
|
|
Cyphostemma (Planch.) Alston (3/200) |
Cyphostemma elephantopus [49], C. laza [31], C. junceum [31] |
|
|
Pseudocayratia J. Wen, L.M.Lu and Z.D.Chen (3/5) |
Pseudocayratia dichromocarpa [50], P. pengiana [50], P. speciosa [50,52] |
|
|
Tetrastigma (Miq.) Planch. (26/100) |
Tetrastigma campylocarpum [51], T. cauliflorum [51], T. caudatum [51], T. delavayi [51], T. dichotomum [52], T. formosanum [51], T. harmandi [31], T. hemsleyanum [31,51], T. henryi [51], T. hypoglaucum [51], T. jinghongense [51], T. kwangsiense [30,31], T. lanceolarium [30], T. laoticum [51], T. obovatum [51,52], T. obtectum [51,52], T. pachyllylum [51], T. pedunculare [31,51,52], T. petraeum [51], T. retinervum [51], T. rumicispermum [31,51,52], T. serrulatum [51], T. sichouense [51], T. thorsborneorum [51], T. triphyllum [31,51], T. xishuangbannaense [31,51] |
|
IV. Parthenocisseae (11/16) |
Parthenocissus Planch. (9/14) |
Parthenocissus dalzielii [36], P. heptaphylla [31], P. henryana [36], P. heterophylla [36], P. himalayana [53], P. laetevirens [36], P. quinquefolia [54], P. tricuspidata [36,37,45], P. vitacea [31] |
|
|
Yua C.L.Li (2/2) |
Yua austro-orientalis [31], Y. chinensis [31] |
|
V. Viteae (32/190) |
Ampelocissus Planch. (13/115) |
Ampelocissus acapulcensis [30], A. bombycina [30], A. bravoi [42], A. cavicaulis [30], A. erdvendbergiana [30], A. grantii [30], A. javalensis [30,42], A. latifolia [30], A. macrocirrha [30], A. martinii [42], A. obtusata [30], A. ochracea [30], A. robinsonii [30] |
|
|
Vitis L. (19/75) |
Vitis aestivalis [55], V. amurensis [45,56], V. brandoniana [54], V. cinerea [57], V. eolabrusca [54], V. flexuosa [54], V. grayensis [58], V. labrusca [45,54,62], V. lanatoides [58], V. latisulcata [58], V. palmata [59], V. pseudorotundifolia [54], V. rostrata [54], V. rotundifolia [31,41,54], V. rupestris [45], V. tiliifolia [60], V. tsoi [31,41], V. vulpina [61,62], V. wilsoniae [31,41] |
Table 3. A summary of groups based on morphological seed types for the analysed species in the Vitaceae. The number of cases found in each group is given between dashes.
|
Group (Geometric Model) |
Examples |
|
Group I (Lenses)-3- |
Cissus quadrangularis [44], C. sterculiifolia [31], Tetrastigma petraeum [51] |
|
Group II (Superellipses)-7- |
Ampelocissus bravoi [42], C. reniformis [31,41], Cyphostemma elephantopus [49], C. laza [31], Tetrastigma campylocarpum [51], T. caudatum [51], T. henryi [51] |
|
Group III (Elongated water drops)-15- |
Ampelopsis arborea [37], Cayratia imerinensis [47], Cissus aralioides [35,40], C. cornifolia [40], C. erosa [43], C. integrifolia [40,42], C. petiolata [40], C. pileata [40], C. populnea [40], C. verticillata [31,41,42,45], C. sciaphila [40], C. smithiana [40], C. willardii [42], Cyphostemma junceum [31], V. vulpina [61,62] |
|
Group IV (Water drops, normal or rounded)-14- |
Ampelopsis bodinieri [36], A. glandulosa [36,39], A. humulifolia [36], Cayratia cheniana [46], Cissus campestris [31,41,42], C. fuliginea [31], C. tuberosa [42], C. granulosa [31], Parthenocissus dalzielii [36], Tetrastigma triphyllum [31,51], Vitis amurensis [45,56], V. labrusca [45,54,62], V. palmata [59], V. rupestris [45] |
|
Group V (Heart curves normal or rounded)-19- |
Ampelopsis aconitifolia [36], A. chaffanjoni [36], A. cordata [38], A. japonica [36], Parthenocissus heptaphylla [31], P. heterophylla [36], P. henryana [36], P. himalayana [51,53], P. quinquefolia [54], P. vitacea [31], P. tricuspidata [36,37,45], Rhoicissus revoilii [31], T. lanceolarium [30], Vitis cinerea [57], V. flexuosa [54], V. lanatoides [58], V. latisulcata [58], V. tsoi [31,41], V. wilsoniae [31,41] |
|
Group VI (Elongated Heart curves)-6- |
Ampelocissus acapulcensis [30], Cissus oligocarpa [31]. V. eolabrusca [54], V. grayensis [58], V. pseudorotundifolia [54], V. tiliifolia [60] |
|
Group VII (Other elongated types)-11- |
Ampelopsis megalophylla [31,36], Causonis sp. [46], Cayratia saponaria [31], Cissus trianae [31], C. hypoglauca [31], Parthenocissus laetevirens [36], T. hypoglaucum [51] Vitis aestivalis [55], V. rotundifolia [31,41,54], Yua austro-orientalis [31], Y. chinensis [31] |
|
Group VIII (Heart curves of the Cayratia and Pseudocayratia types)-7- |
Cayratia japonica [31,48], Cayratia sp. [African, 46], Pseudocayratia dichromocarpa [50], P. pengiana [50], P. speciosa [50,52], Tetrastigma formosanum [51], T. pedunculare [31,51,52] |
|
Group IX (Heart curves of the SqHC type of Ampelocissus and Ampelopsis)-15- |
Ampelocissus bombycina [30], A. cavicaulis [30], A. erdvendbergiana [30], A. grantii [30], A. javalensis [30,42], A. latifolia [30], A. macrocirrha [30], A. martinii [42], A. obtusata [30], A. ochracea [30], A. robinsonii [30], Ampelopsis cantoniensis [31,36], A. delavayana [31], A. denudata [30], A. grossedentata [31] |
|
Group X Elongated Superellipse-heart curves-16- |
Cissus elongata [40], C. penninervis [31], Rhoicissus rhomboidea [31], Tetrastigma hemsleyanum [31,51], T. jinghongense [51], T. laoticum [51], T. cauliflorum [51], T. dichotomum [52], T. harmandi [31], T. pachyllylum [51], T. kwangsiense [30,31], T. obovatum [51,52], T. obtectum [51,52], T. retinervum [52], T. serrulatum [51], T. sichouense [51] |
|
Undefined-18- |
Cayratia geniculata [31], Cissus antarctica [31], C. barbeyana [40], C. bosseri [40], C. cactiformis [40], C. descoingsii, [31,41], C. diffusiflora [40], C. floribunda [40], C. hastata [40], C. leucophlea [40], C. repens [40], C. subtetragona [40], T. delavayi [51], T. rumicispermum [31,51,52], T. thorsborneorum [51], T. xishuangbannaense [31,51], V. brandoniana [54], V. rostrata [54] |
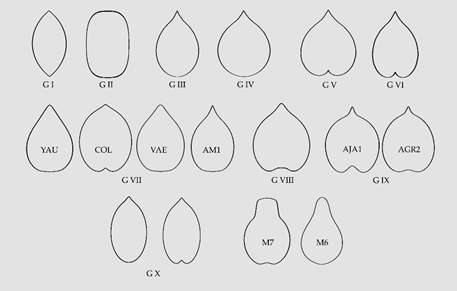
Figure 1. A summary of the models found for the description and quantification of seed shape in the Vitaceae. G I, G II and G III are lenses, superellipses and elongated water drops, respectively; G IV, G V and G VI correspond to water drops, heart curves and elongated heart curves , respectively; G VII contains four models corresponding to other elongated curves; G VIII presents an example of the heart curves of the Cayratia and Pseudocayratia types; G IX, heart curves of the Squared Heart Curves (SqHCs) type in Ampelocissus and broadened models of Ampelocissus and Ampelopsis, and G X, Elongated Superellipse-Heart Curves (ESHCs), frequent in Tetrastigma species and observed also in Cissus species and R. rhomboidea. Labelled as M7 and M6 are two models used in the description of seeds of grape varieties and as precursors for other models[27][28].
In general, the distribution of morphological types is not in close agreement with the current taxonomic classification; nevertheless, some results may be summarized in this aspect. First, the seeds of the Elongated Superellipse-Heart Curves (ESHCs) type (Group X) are more frequent in Tetrastigma and have been observed in Rhoicissus and Cissus, but not in species of other genera. While many seeds in species of Ampelopsis, Parthenocissus and Vitis share the typical shapes of water drop and heart curves, the squared heart curve (SqHC) type (Group IX) has been predominantly observed in Ampelocissus and Ampelopsis. A number of species remain undefined due to one of these two reasons: First, their irregular seed shape making difficult the identification of an adequate model (Cayratia geniculata, Cissus antarctica) and, second, the seed images have geometric shapes but the identification of the model with the corresponding equation is pending (Tetrastigma delavayi, T. rumicispermum). In addition, further work will be done on the seeds of Vitis species.
This entry is adapted from the peer-reviewed paper 10.3390/plants10081695
References
- Zhang, N.; Wen, J.; Zimmer, E.A.; Congruent deep relationships in the grape family (Vitaceae) based on sequences of chloroplast genomes and mitochondrial genes via genome skimming. PLoS ONE 2015, 10, e0144701, .
- Zeng, L.P.; Zhang, N.; Zhang, Q.; Endress, P.K.; Huang, J.; Ma, H.; Resolution of deep eudicot phylogeny and their temporal diversification using nuclear genes from transcriptomic and ge-nomic datasets. New Phytol. 2017, 214, 1338–1354, 10.1111/nph.14503.
- 3. Wen, J.; Lu, L.-M.; Nie, Z.-L.; Liu, X.-Q.; Zhang, N.; Ickert-Bond, S.; Gerrath, J.; Manchester, S.R.; Boggan, J.; Chen, Z.-D.; et al. A new phylogenetic tribal classification of the grape family (Vitaceae). J. Syst. Evol. 2018, 56, 262–272, .
- Rossetto, M.; Jackes, B.R.; Scott, K.D.; Henry, R.J.; Is the genus Cissus (Vitaceae) monophyletic? Evidence from Plastid and Nuclear Ribosomal DNA. Syst. Bot. 2002, 27, 522–533, 10.1043/0363-6445-27.3.522.
- Hearn, D.J.; Evans, M.; Wolf, B.; McGinty, M.; Wen, J; Dispersal is associated with morphological innovation, but not increased diversification, in Cyphostemma (Vitaceae). J. Syst. Evol. 2018, 56, 340–359, 10.1111/jse.12417.
- Terral, J.F.; Tabard, E.; Bouby, L.; Ivorra, S.; Pastor, T.; Figueiral, I.; Picq, S.; Chevance, J.-B.; Jung, C.; Fabre, L.; et al. Evolution and history of grapevine (Vitis vinifera) under domestication: New morphometric perspectives to understand seed domestication syndrome and reveal origins of ancient European cultivars. Ann. Bot. 2010, 105, 443–455, 10.1093/aob/mcp298.
- This, P.; Lacombe, T.; Thomas, M.R.; Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519, 10.1016/j.tig.2006.07.008.
- Gutiérrez del Pozo, D.; Martín-Gómez, J.J.; Tocino, Á.; Cervantes, E.; Seed geometry in the Arecaceae. Horticulturae 2020, 6, 64, 10.3390/horticulturae6040064.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W.; NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675, http://imagej.nih.gov/ij/docs/guide .
- Cervantes, E.; Martín-Gómez, J.J.; Saadaoui, E.; Updated methods for seed shape analysis. Scientifica 2016, 5691825, 5691825, 10.1155/2016/5691825.
- Cervantes, E.; Martín-Gómez, J.J.; Seed shape description and quantification by comparison with geometric models. Horticulturae , 2019, 5, 60, doi:10.3390/horticulturae5030060.
- Cervantes, E.; Martín-Gómez, J.J.; Gutiérrez del Pozo, D.; Silva Días, L.; An angiosperm species dataset reveals relationships between seed size and two-dimensional shape. Horticulturae 2019, 5, 71, horticulturae5040071.
- Cervantes, E.; Martín-Gómez, J.J.; Ardanuy, R.; de Diego, J.G.; Tocino, Á.; Modeling the Arabidopsis seed shape by a cardioid: Efficacy of the adjustment with a scale change with factor equal to the Golden Ratio and analysis of seed shape in ethylene mutants. J. Plant. Physiol. 2010, 167, 408–410, doi:10.1016/j.jplph.2009.09.013.
- Martín Gómez, J.J.; Tocino, Á.; Ardanuy, R.; de Diego, J.G.; Cervantes, E.; Dynamic analysis of Arabidopsis seed shape reveals differences in cellulose mutants. Acta Physiol. Plant. 2014, 36, 1585–1592, .
- Cervantes, E.; Martín-Gómez, J.J.; Chan, P.K.; Gresshoff, P.M.; Tocino, Á.; Seed shape in model legumes: Approximation by a cardioid reveals differences in ethylene insensitive mutants of Lotus ja-ponicus and Medicago truncatula. . J. Plant. Physiol. 2012, 169, 1359–1365, j.jplph.2012.05.019.
- Saadaoui, E.; Martín-Gómez, J.J.; Cervantes, E.; Intraspecific variability of seed morphology in Capparis spinosa L. . Acta Biol. Cracov. Bot. 2013, 55, 99–106, .
- Saadaoui, E.; Martín-Gómez, J.J.; Tlili, N.; Khaldi, A.; Cervantes, E.; Effect of climate in seed diversity of wild Tunisian Rhus tripartita (Ucria) Grande. J. Adv. Biol. Biotechnol. 2017, 13, 1–10, 10.9734/JABB/2017/32786.
- Martín-Gómez, J.J.; Rewicz, A.; Rodríguez-Lorenzo, J.L.; Janoušek, B.; Cervantes, E.; Seed morphology in Silene based on geometric models. Plants 2020, 9, 1787, 10.3390/plants9121787.
- Martín-Gómez, J.J.; Saadaoui, E.; Cervantes, E; Seed shape of castor bean (Ricinus communis L.) grown in different regions of Tunisia. . J. Agric. Ecol. Res. Int. 2016, 8, 1–11, .
- Saadaoui, E.; Martín, J.J.; Bouazizi, R.; Chokri, B.R.; Grira, M.; Abdelkabir, S.; Khouja, M.L.; Cervantes, E.; . Phenotypic variability and seed yield of Jatropha curcas L. introduced to Tunisia. Acta Bot. Mex. 2015, 110, 119–134, .
- Martín-Gómez, J.J.; Rewicz, A.; Goriewa-Duba, K.; Wiwart, M.; Tocino, Á.; Cervantes, E.; Morphological description and classification of wheat kernels Based on geometric models. Agronomy 2019, 9, 399, 10.3390/agronomy9070399.
- Cervantes, E.; Martín-Gómez, J.J.; Seed shape quantification in the order Cucurbitales. Modern Phytomorphol. 2018, 12, 1–13, 10.5281/zenodo.117487.
- Martín-Gómez, J.J.; Rewicz, A.; Cervantes, E.; Seed shape diversity in families of the order Ranunculales. Phytotaxa 2019, 425, 193–207, 10.11646/phytotaxa.425.4.1 .
- Martín-Gómez, J.J.; Gutiérrez del Pozo, D.; Cervantes, E.; Seed shape quantification in the Malvaceae reveals cardioid-shaped seeds predominantly in herbs.. Bot. Lith. 2019, 25, 21–31, 10.2478/botlit-2019-0003.
- Chen, I.; Manchester, S.R.; Seed morphology of modern and fossil Ampelocissus (Vitaceae) and implications for phytogeography. Am. J. Bot. 2007, 94, 1534–1553, 10.3732/ajb.94.9.1534..
- Chen, I.; Manchester, S.R.; Seed morphology of modern and fossil Ampelocissus (Vitaceae) and implications for phytogeography. Am. J. Bot. 2007, 94, 1534–1553, 10.3732/ajb.94.9.1534..
- Martín-Gómez, J.J.; Gutiérrez del Pozo, D.; Ucchesu, M.; Bacchetta, G.; Cabello Sáenz de Santamaría, F.; Tocino, Á.; Cervantes, E.; Seed morphology in the Vitaceae based on geometric models. Agronomy 2020, 10, 739, 10.3390/agronomy10050739.
- Cervantes, E.; Martín-Gómez, J.J.; Espinosa-Roldán, F.E.; Muñoz-Organero, G.; Tocino, Á.; Cabello-Sáenz de Santamaría, F.; Seed morphology in key Spanish grapevine cultivars. Agronomy 2021, 11, 734, 10.3390/agronomy11040734.
