2. Neutrophil Contributions to Lymphangiogenesis
Emerging evidence suggests that neutrophils may also interact with the lymphatic system by regulating lymphangiogenesis (or growth of new lymphatic vessels) in inflamed tissues and lymph nodes during inflammation and cancer progression [
3,
95]. Recent studies have identified signals which promote the formation of the lymphatic vasculature [
96,
97]. Vascular endothelial growth factors, such as VEGF-A, VEGF-C and VEGF-D can induce LEC sprouting and proliferation. VEGFs bind with different specificity to three endothelial transmembrane receptors: VEGFR-1, VEGFR-2, and VEGFR-3. The best characterized axis in lymphatic expansion and development involves VEGFR-3 and its ligands, VEGF-C and VEGF-D [
98].
The functional consequences of inflammation-induced lymphangiogenesis require further investigation. In some settings, expansion of the lymphatic network may support inflammation by promoting the transport of immune cells and antigens to the lymph node and enhancing the immune response. In other settings, lymphatic vessel expansion contributes to resolution of inflammation by draining inflammatory mediators and cells from the site of inflammation [
99]. For instance, inhibiting the lymphatic vasculature led to increased inflammation in mouse models of skin inflammation [
100,
101], inflammatory bowel disease [
102,
103] and rheumatoid arthritis [
104]. However, inhibiting this process improves graft survival in corneal transplantation [
105,
106].
Unlike the well-established role for neutrophils in promoting blood vessel formation (angiogenesis) by producing VEGFs [
107], neutrophil contribution to lymphangiogenesis is only just beginning to be uncovered [
22]. Neutrophils secrete VEGF-D at sites of inflammation, which promotes proliferation of LECs, stimulates lymphangiogenesis [
108] and plays an important role during the development of the lymphatic system [
109]. Neutrophils may also increase VEGF-A bioavailability [
18]. In fact, after secretion VEGF becomes bound to the ECM and the interaction between VEGF-A and matrix proteins is mediated through the carboxy terminal region, also known as the heparin-binding or ECM-binding domain [
110]. This domain is able to bind heparin sulphate proteoglycans and other matrix proteins [
110,
111] and is thought to be responsible for the sequestration of VEGF-A within the matrix. Neutrophils act via MMP-9 and heparanase (which cleaves heparan sulphate preoteoglycans (HSP) side chains and releases the HSP-trapped VEGF-A) (
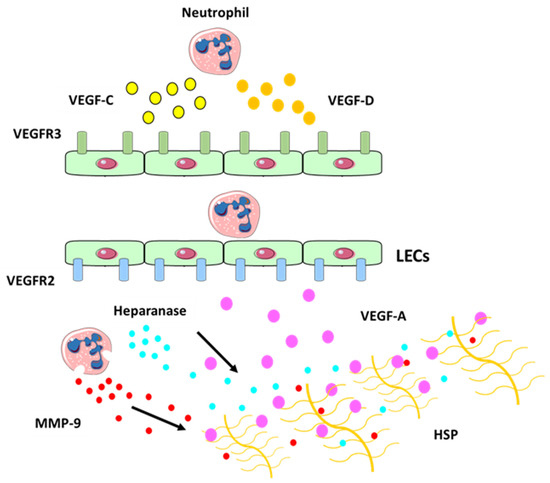
Figure 4) to increase the amount of biologically active VEGF-A, which in turn activates inflammatory lymphangiogenesis [
18].
Figure 4. Neutrophil contribution to inflammatory lymphangiogenesis. Schematic illustrating neutrophil contributions to lymphangiogenesis at the site of inflammation. Neutrophils can secrete VEGF-C (in yellow) and VEGF-D (in orange) at the site of inflammation which bind to their receptor VEGFR-3 (in green) expressed on LECs. Neutrophils can also increase VEGF-A (in purple) availability by secreting MMP-9 and heparanase which cleaves heparan sulphate preoteoglycans (HSP) side chains and release the HSP-trapped VEGF-A. This binds to VEGFR-2 receptor (in blue), which promotes the expansion of the lymphatic vessel network [
119].
Neutrophils may also promote tissue lymphangiogenesis by cooperating with macrophages [
18,
112], as both macrophages and neutrophils contributed to inflammatory lymphangiogenesis in zebrafish by expressing VEGF-A, VEGF-C and VEGF-D [
113]. Neutrophils also expressed VEGF-D in the mucosa and lumen of inflamed airways [
17]. Furthermore, a study of lymphangiogenesis in abdominal aortic aneurysm (AAA) reported neutrophil infiltration around lymphatic microvessels [
114].
Similarly to tissue lymphangiogenesis, VEGF-A-VEGFR2 and VEGF-C/D-VEGFR3 are the principal mediators of lymph node (or intranodal) lymphangiogenesis [
115]. During inflammation, B cells [
116], macrophages recruited from peripheral tissues [
117] and fibroblast-type reticular stromal cells [
118] are the major sources of VEGF-A in the lymph node. Emerging evidence suggests that neutrophils also contribute to lymph node lymphangiogenesis [
18]. In mice lacking B cells, neutrophils compensate to support lymph lymphangiogenesis during prolonged inflammation [
18]. However, more studies are needed to determine whether specific neutrophil subsets are involved in this process.
Together, these studies support the emerging concept of neutrophils as important contributors to lymphangiogenesis in inflamed tissues and draining lymph nodes via the secretion of lymphangiogenic factors and LEC stimulation during the early and later stages of inflammation.