Venous thromboembolism, which includes both deep venous thrombosis and pulmonary embolism, is a major cause of morbidity and mortality among patients with cancer. The impact of ABO blood type in the development of venous thromboembolism in cancer patients remains controversial. To develop a sense of current opinion in this area, we conducted a systematic review and meta-analysis. Blood type is routinely determined preoperatively by objective and standardized methods, and our results suggest that these blood type results are useful for risk stratification and potentially for encouraging appropriate strategies for implementation of prophylactic treatment strategy in venous thromboembolism management.
- ABO blood type
- venous thromboembolism
- meta-analysis
1. Introduction
2. Study Selection
3. Characteristics of the Included Studies
| VTE | |||||||
|---|---|---|---|---|---|---|---|
| First Author of Study and [Ref.] | Country | Recruitment Period | Study Design | Total | Yes | No | NOS |
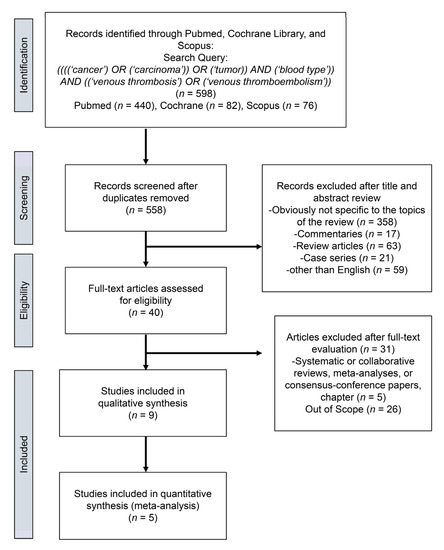
| Streiff et al. [8] | USA | 1991–2001 | Cohort, retrospective | 130 | 28 | 102 | 6 |
| Tollefson et al. [9] | USA | 1987–2010 | Cohort, retrospective | 18472 | 271 | 18201 | 7 |
| Wang J et al. [10] | USA | 1980–2005 | Cohort, retrospective | 2076 | 216 | 2060 | 7 |
| Mizrahi et al. [11] | Canada | 1995–2013 | Cohort, retrospective | 523 | 56 | 467 | 6 |
| Li et al. [12] | USA | NR | Cohort, retrospective | 670 | 236 | 434 | 6 |
| Spavor et al. [13] | Canada | NR | Cohort, retrospective | 218 | 63 | 155 | 6 |
| Bhanvadia et al. [14] | USA | 2003–2015 | Cohort, retrospective | 1341 | 90 | 1251 | 7 |
| Heenkenda et al. [15] | Sweden | NR | Cohort, retrospective | 139 | 47 | 92 | 6 |
| Wang G et al. [16] | China | 2018–2019 | Cohort, retrospective | 2315 | 131 | 2174 | 7 |
4. Meta-Analysis
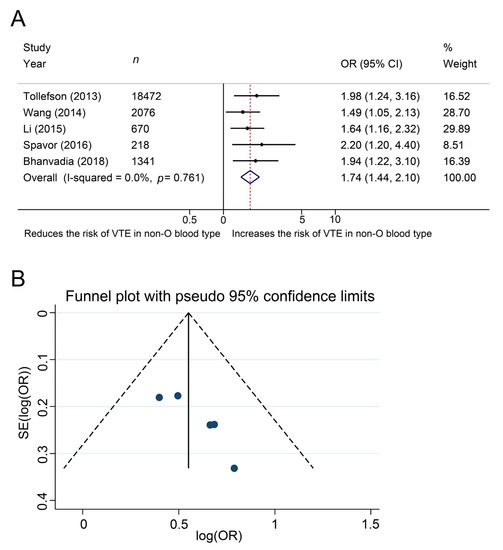
5. Additional Analysis
This entry is adapted from the peer-reviewed paper 10.3390/jcm10163692
References
- Park, B.; Messina, L.; Dargon, P.; Huang, W.; Ciocca, R.; Anderson, F.A. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: Findings from the nationwide inpatient sample. Chest 2009, 136, 983–990.
- Nakchbandi, I.A.; Löhr, J.-M. Coagulation, anticoagulation and pancreatic carcinoma. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 445–455.
- Agnelli, G.; Bolis, G.; Capussotti, L.; Scarpa, R.M.; Tonelli, F.; Bonizzoni, E.; Moia, M.; Rossi, R.; Sonaglia, F.; Valarani, B.; et al. A clinical outcome-based prospective study on venous thromboembolism in cancer surgery: The @RISTOS project. Ann. Surg. 2006, 243, 89–95.
- Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Bill-Axelson, A.; Carlsson, S. Thromboembolic events following surgery for prostate cancer. Eur. Urol. 2013, 63, 354–363.
- Heit, J.A.; Cunningham, J.M.; Petterson, T.M.; Armasu, S.M.; Rider, D.N.; De Andrade, M. Genetic variation within the anticoagulant, procoagulant, fibrinolytic and innate immunity pathways as risk factors for venous thromboembolism. J. Thromb. Haemost. 2011, 9, 1133–1142.
- Dentali, F.; Sironi, A.P.; Ageno, W.; Turato, S.; Bonfanti, C.; Frattini, F.; Crestani, S.; Franchini, M. Non-O blood type is the commonest genetic risk factor for VTE: Results from a meta-analysis of the literature. Semin. Thromb. Hemost. 2012, 38, 535–548.
- Kabrhel, C.; Varraso, R.; Kraft, P.; Rimm, E.B.; Goldhaber, S.Z.; Camargo, C.; Fuchs, C.S.; Wolpin, B.M. Prospective study of ABO blood type and the risk of pulmonary embolism in two large cohort studies. Thromb. Haemost. 2010, 104, 962–971.
- Streiff, M.B.; Segal, J.; Grossman, S.A.; Kickler, T.S.; Weir, E.G. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer 2004, 100, 1717–1723.
- Tollefson, M.K.; Karnes, R.J.; Rangel, L.; Carlson, R.; Boorjian, S.A. Blood type, lymphadenectomy and blood transfusion predict venous thromboembolic events following radical prostatectomy with pelvic lymphadenectomy. J. Urol. 2014, 191, 646–651.
- Wang, J.K.; Boorjian, S.A.; Frank, I.; Tarrell, R.F.; Thapa, P.; Jacob, E.K.; Tauscher, C.D.; Tollefson, M.K. Non-O blood type is associated with an increased risk of venous thromboembolism after radical cystectomy. Urology 2014, 83, 140–145.
- Mizrahi, T.; Leclerc, J.-M.; David, M.; Ducruet, T.; Robitaille, N. ABO group as a thrombotic risk factor in children with acute lymphoblastic leukemia: A retrospective study of 523 pediatric patients. Blood 2013, 122, 2370.
- Li, D.; Pise, M.N.; Overman, M.J.; Liu, C.; Tang, H.; Vadhan-Raj, S.; Abbruzzese, J.L. ABO non-O type as a risk factor for thrombosis in patients with pancreatic cancer. Cancer Med. 2015, 4, 1651–1658.
- Spavor, M.; Halton, J.; Dietrich, K.; Israels, S.; Shereck, E.; Yong, J.; Yasui, Y.; Mitchell, L.G. Age at cancer diagnosis, non-O blood group and asparaginase therapy are independently associated with deep venous thrombosis in pediatric oncology patients: A risk model. Thromb. Res. 2016, 144, 27–31.
- Bhanvadia, S.; Kazerouni, K.; Bazargani, S.T.; Miranda, G.; Cai, J.; Daneshmand, S.; Djaladat, H. Validating the role of ABO blood type in risk of perioperative venous thromboembolism after radical cystectomy. World J. Urol. 2018, 37, 173–179.
- Heenkenda, M.K.; Malmström, A.; Lysiak, M.; Mudaisi, M.; Bratthäll, C.; Milos, P. Assessment of genetic and non-genetic risk factors for venous thromboembolism in glioblastoma—The predictive significance of B blood group. Thromb. Res. 2019, 183, 136–142.
- Wang, G.; Wang, H.; Shen, Y.; Dong, J.; Wang, X.; Wang, X.; Zheng, Y.; Guo, S. Association between ABO blood group and venous thrombosis related to the peripherally inserted central catheters in cancer patients. J. Vasc. Access 2020, 2020.
