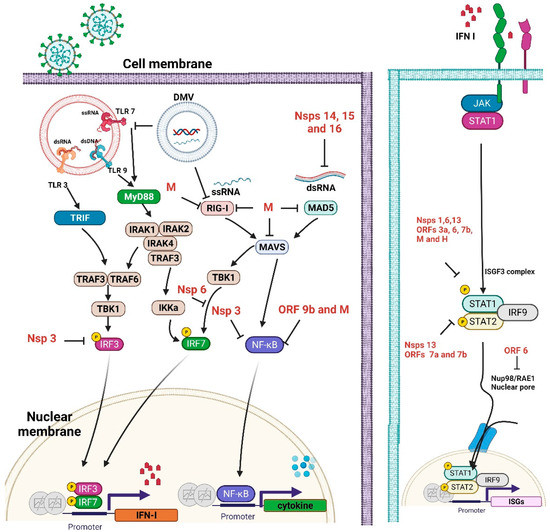
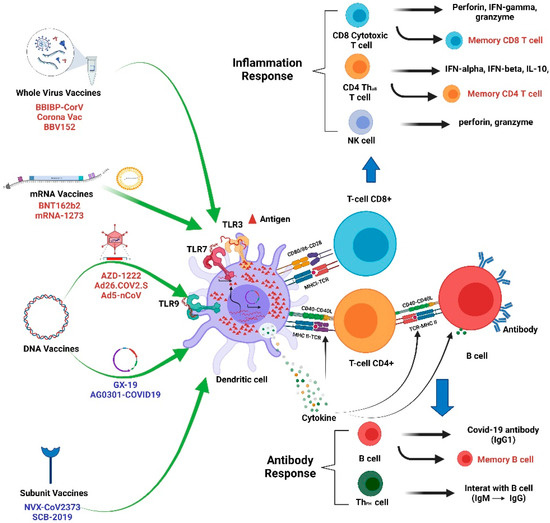
5. Possible Links between Vitamin D and Vaccine Effectiveness
At present, significant efforts have been made to develop effective and safe vaccines for SARS-CoV-2, resulting in the development of inactivated vaccines, DNA/mRNA vaccines, and protein subunit vaccines [
6]. However, the role of vitamin D in the effectiveness of these vaccines has not yet been confirmed by further studies.
Vitamin D deficiency (VDD) occurs all over the world, mainly in the Middle East, China, Mongolia, and India [
94]. The question of whether VDD affects immune responses to influenza immunization is inconclusive. Seroprotection (SP) rates of subtype H3N2 (A/H3N2) and strain B of influenza A virus in VDD patients are lower than those of patients with normal levels of vitamin D [
95]. Vitamin D deficiency is prevalent among COVID-19 patients. A study based on the Israeli population showed that a low level of vitamin D in plasma of 25(OH) is associated with a higher risk of COVID-19 infection [
96]. Low levels of 25(OH)D at hospitalization have been associated with the COVID-19 stage and mortality [
97]. Correlations have been observed between the historical prevalence of vitamin D deficiency and COVID-19 mortality in European countries [
98]. The amazingly high levels of vitamin D in Scandinavian countries reflect their policy of vitamin D fortification and supplementation [
99]. Systematic vitamin D food fortification is an effective approach to improve vitamin D deficiency in the general population and has already been introduced by countries such as the U.S., Canada, and Finland [
100].
Currently, dark skin color, age, pre-existing conditions, and vitamin D deficiency are characteristics of patients with severe COVID-19. Among these, only vitamin D deficiency can be modified. Numerous observational studies have provided evidence that serum 25-hydroxyvitamin D levels are inversely correlated with the incidence and severity of COVID-19. These observations support our hypotheses. This evidence to date generally satisfies Hill’s criteria for causality in a biological system, such as strength of association, consistency, temporality, biological gradient, plausibility, and coherence, although experimental verification is lacking [
101].
Experience in the development of SARS-CoV vaccines has raised concerns about the correlation between pulmonary histopathology and immune responses to Th2 cytokines [
102]. Th2 cells can secrete many cytokines, such as IL-4, IL-5, IL-10, and IL-13. Aberrantly high levels of Th2 cytokines can elicit immune responses that prompt eosinophilic infiltrations. Four different SARS-CoV vaccines led to the development of Th2-type immunopathology with elevated eosinophilic infiltration, which represented a Th2-type hypersensitivity marker in mouse models [
103]. Similar results were observed in inactivated MERS-CoV vaccines that also showed eosinophilic infiltration, with IL-5 and IL-13 levels higher than those that existed before vaccination in mouse models [
104] content-type="background:white">. The immune response after vaccination can be partially attributed to the presence of the nucleocapsid (N) protein in the vaccine [
105]. Studies of cytokine characteristics in patients infected with SARS-CoV-2 also showed an increase in Th2 cytokine secretion, which could contribute to lung histopathology [
106]. Therefore, the control of the T-cell response should be considered in the development of SARS-CoV-2 vaccines. Proper vitamin D supplementation can mitigate the inflammatory effects of the COVID-19 vaccine.
The vaccine-induced humoral immune response may reflect effective protection against SARS-CoV-2 infection. However, the reaction to aberrant antibodies could have adverse effects in some patients [
6]. In SARS-CoV-infected animal models, vaccine-induced S-specific IgG can cause severe acute pulmonary injury since these IgG antibodies disrupted the inflammation resolution response with the blocking of Fc gamma receptor (FcγR) on the cell membrane of activated macrophages [
107]. During the acute phase, deceased patients usually display higher levels of neutralizing antibodies (NAbs), which decrease more rapidly than in recovered patients. This reflects the potentially systematic breakdown of the immune system, which causes pathological pulmonary effects [
107,
108]. Consistently, patients with severe SARS-CoV-2 infections frequently experience significant IgG3 reactions, which were linked to the worst clinical condition with the antibody-dependent enhancement (ADE) of COVID-19 [
109]. It is currently unclear whether SARS-CoV-2 vaccines will induce an aberrant reaction to antibodies, and further research is needed to explore potential lung damage from SARS-CoV-2 vaccines. We speculate that appropriate vitamin D supplementation can promote immunity through acceleration and cooperation with IFN-I, promoting the production of antibodies from B cells that are dependent on the T cells of the COVID-19 vaccine.
Age is known to affect vaccine immunity. Vaccinated aged animals that were challenging to immunize also displayed eosinophilic infiltration in the lungs. Neutralizing antibody titers were significantly reduced in aged vaccinated groups compared to young groups [
110]. In brief, elderly populations with underlying diseases, including diabetes, hypertension, and cardiovascular disease, are at high risk for vitamin D deficiency and COVID-19 [
111]. Given the severity of the disease in elderly people, older animal models are essential for the preclinical validation of vaccines. Even patients on maintenance hemodialysis developed a substantial humoral response following the BNT162b2 vaccine, although it was significantly lower than that of controls. Age was an important factor in the humoral response, regardless of chronic medical conditions [
112]. Vit-D and the VDR pathway both have an important anti-inflammatory function, and the lack of vit-D in aged subjects likely increases the risk of chronic mild inflammation conditions [
113], resulting in poor responses to vaccines.
Two clinical studies have been presented to explain the potential benefits of vitamin D supplementation for vaccine efficacy. ChAdOx1 nCoV-19 (AZD1222) is a candidate SARS-CoV-2 vaccine comprising a replication-deficient simian adenovirus expressing the whole SARS-CoV-2 spike protein. The vaccine was tolerated, and antigen-specific neutralizing antibodies and T lymphocytes were induced against the SARS-CoV-2 spike protein. Eight weeks after a single-dose vaccination, adults demonstrated an induced S-protein-reactive CD4
+ T with a T helper (THαβ)-type cytokine bias and CD8
+ T cells with a cytotoxic phenotype. These are important findings, as THαβ-type immunity is believed to facilitate protective antiviral immunity. Robust B-cell activation and proliferation were also observed, and IgG of anti-S proteins (mainly the IgG1 isotype) were detected from day 14 to day 56. In particular, these antibodies demonstrated neutralizing activity against SARS-CoV-2, and their affinity for the S protein increased from day 28 to day 56. A single vaccination also gave rise to IgM and IgA antibodies specific to the S protein [
114]. Besides the antibody titer elevation, ChAdOx1 nCoV-19 vaccination also increased IgG antibody avidity significantly to provide seroprotection. Adequate vit-D can aid in THαβ-type immunity and promote the activation of B cells with higher levels of IgG-neutralizing antibodies. Further investigation of a booster dose of ChAdOx1 nCoV-19 found it to be safe and more tolerable than initiation doses. A study shows that a second vaccination improves the titers of anti-S antibodies and the neutralizing activity, which promotes THαβ-type T-cell responses. Moreover, the booster dose further enhances the functional capacity of anti-S antibodies to support antibody-dependent cellular cytotoxicity, complement deposition, and natural killer cell activation. These have been linked with protective immunity in preclinical studies [
115]. All these responses could be accentuated in the presence of vit-D adequacy. Importantly, the second dose of the vaccine was shown to be safe and better tolerated than the first dose. Since the majority of COVID-19 candidate vaccines are designed to target the SARS-CoV-2 spike protein, it remains to be determined whether the specific immunity correlates with vaccine-mediated protection. Thus, this two-dose vaccine regimen is more effective in promoting immunity to SARS-CoV-2 and is well tolerated. These data also suggest that the booster dose should remain effective if administered eight to twelve weeks after the initial vaccination [
116]. Similar results should also be noted in other vaccines to show the link between vit-D adequacy and seroconversion or sero-protectivity because vit-D can aid the activation of antiviral THαβ-type immunity.
Better vitamin D status was shown to improve seroconversion in response to influenza vaccinations [
82]. The control of the current COVID-19 pandemic and mortality is likely to be highly dependent on effective vaccination, but vitamin D deficiency continues to be common across the U.K. and other nations. Better vitamin D status was also associated with reductions in COVID-19 risks in a prospective study in the USA. Vitamin D supplement is related to a reduction in acute viral respiratory infection. In addition, studies suggest that better vitamin D supplementation in order to correct the deficiency with the metabolite calcifediol can reduce COVID-19 severity and mortality. Inadequate vitamin D serum level is related to COVID-19 incidence, severity, and mortality. The low vitamin D level is shown to be an independent risk factor of SARS-CoV-2 infection and COVID-19 hospitalization. Individuals with vitamin D deficiency tend to have more severe symptoms of SARS-CoV-2 infection. As a result of this information, the correction of vitamin D deficiency is included in the clinical management for the treatment of COVID-19 patients. A recent report using U.K. Biobank data found a strong inverse association of serum 25(OH)D values with COVID-19 severity [
117,
118].
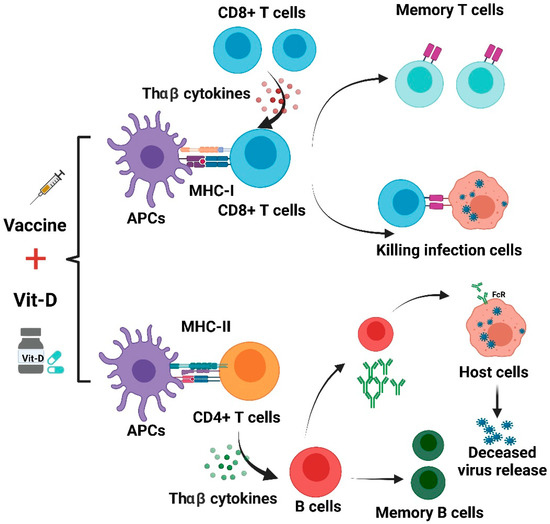
We suggest that an intake of vitamin D to reduce the rate of deficiency could provide a simple, safe, and cheap aid in reducing COVID-19 risks. If protection afforded by vaccinations against COVD-19 proved to be increased through the repletion of pre-existing vitamin D deficiencies, these effects would be useful adjunctive measures for reducing COVID-19 risks globally, especially in high-risk groups for COVID-19. A summary of Vit-D effect on COVID-19 vaccines is shown in Figure 3.
Figure 3. Effects of vitamin D on immune responses induced by COVID-19 vaccines.
The use of vitamin D supplements may improve immune responses from different COVID-19 vaccines. As shown in
Figure 3, antigen-presenting cells (APCs) treat the vaccine as an antigen and then present it to CD8+ T and CD4+ T cells. CD8+ T lymphocytes can be activated by THαβ cytokines and acquire the capacity to attack infected cells. This process could be complemented with appropriate vitamin D supplementation. THαβ cytokines can aid in the differentiation of B cells. The activated B cells are able to produce NAbs. Vit-D can also improve antibody generation in a T-cell-dependent B-cell manner. However, unbalanced immune responses can lead to lung immunopathology induced by aberrant ADE, and this may also be mitigated by treatment using vit-D [
6,
114].