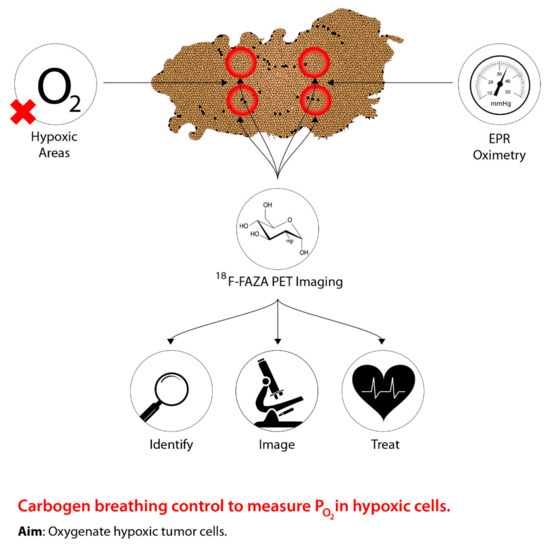
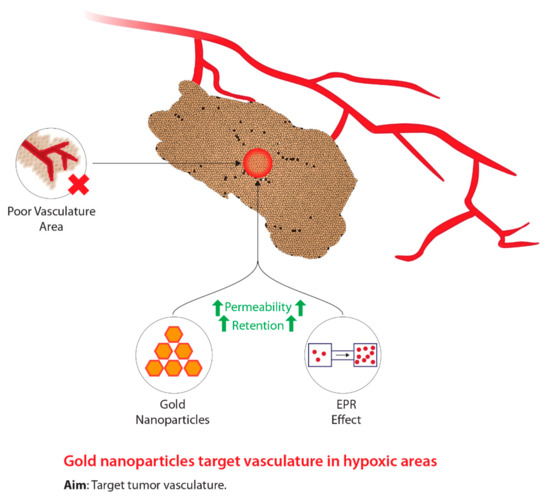
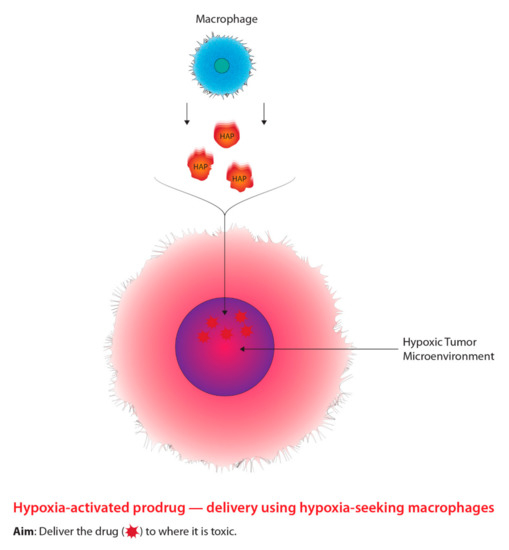
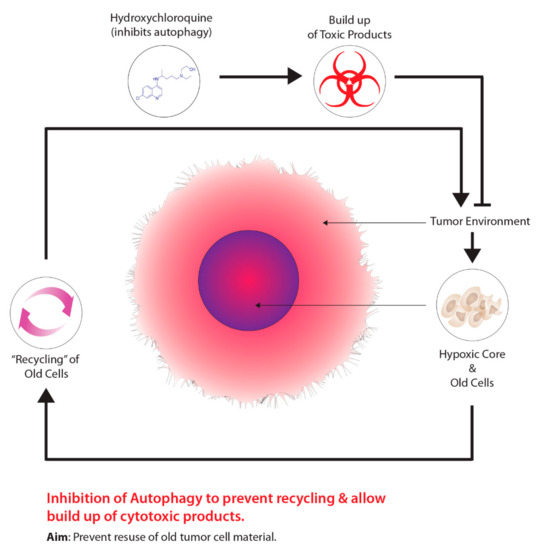
As previously stated, in order to facilitate effective tumour hypoxia imaging, an “ideal” PET radiotracer is required and must meet a number of biochemical characteristics [
28]. Although many compounds are being investigated, virtually all compounds do not meet all the criteria of the “ideal” PET tracer, nor are they available for imaging all tumour types [
29]. Despite the lack of an “ideal” PET tracer, research has been focused on nitroimidazole analogs, specifically, 2-nitroimidazole [
28]. Although nitroimidazoles were originally intended to be radiosensitizers, Chapman et al. [
30] demonstrated that these compounds can serve as hypoxia markers. These compounds are able to passively diffuse into cells, which is largely determined by the intracellular environment [
31]. The main driver of the initial reduction following passive diffusion of nitroimidazoles is the concentration of intracellular oxygen [
31]. Once the compounds have entered the cell, nitroimidazoles will undergo single-electron reduction to create a free radical anion [
31]. Subsequently, within normoxic cells, free radical anions are promptly reoxidized to the parent compound through intracellular oxygen levels due to the high electron affinity relative to the nitro group on nitroimidazole [
31]. In contrast, following single-electron reduction of nitroimidazole within hypoxic tumour cells, due to low intracellular oxygen concentrations, reoxidation cannot be completed [
31]. Subsequently, incomplete reoxidation results in the further reduction of the free radical anion, creating a very reactive species that are able to bind to components of a cell [
31,
32]. Furthermore, reduced nitroimidazole has been shown to accumulate within hypoxic cells, thus, demonstrating its potential as a PET tracer [
28]. Of the nitroimidazole analogs screened as PET tracers, most compounds are fluorinated nitroimidazoles; however,
18F-fluoromisonidazole (
18F-FMISO) has garnered the most success and has been extensively studied [
28]. The mechanism of nitroimidazole analogs entrapment within hypoxic tumour cells can be applied to
18F-FMISO.
18F-FMISO is a lipophilic compound; thus, it is readily available to passively diffuse into cells, subsequently, reduction of this compound via the nitroreductase enzyme (NTR), results in the production of R-NO
2 radicals [
33]. Furthermore, due to the low intracellular oxygen levels (pO
2 < 10 mmHg), these radicals are unable to be reoxidized, leading to further reduction of R-NO
2 radicals to form R-NHOH molecules that can bind to cellular components, allowing for tumour hypoxia imaging [
33,
34,
35,
36]. Recently, another type of fluorinated nitroimidazole,
18F-Fluoroazomycin arabinoside (
18F-FAZA), has been gaining more popularity relative to
18F-FMISO [
33]. Studies suggest that
18F-FAZA, in comparison to
18F-FMISO, is less lipophilic due to the presence of an additional sugar moiety [
33,
37,
38]. Moreover, due to structural composition differences,
18F-FAZA has a faster diffusion and clearance rate relative to
18F-FMISO, allowing for an improved tumour-to-background (T/B) ratio, thus, this compound has been gaining more interest as a PET radiotracer [
39,
40]. Overall, due to the new spotlight on
18F-FAZA as a PET radiotracer for tumour hypoxia imaging, researchers are beginning to investigate the use of PET imaging to mediate oxygen-based manipulation therapies, such as carbogen breathing. Thus, tumour hypoxia imaging serves as a promising method to identify hypoxic tumour microenvironments relative to normal tissue, ultimately improving the efficacy of oxygen-based manipulation therapies. In relation to the non-targeted effect theme of this review, which is discussed in detail later, it is likely that increased oxygen will increase oxidative stress and lead to initiation of both bystander signalling and genomic instability phenotypes. To what extent this impacts the therapeutic ratio is not known and is an area for further research.