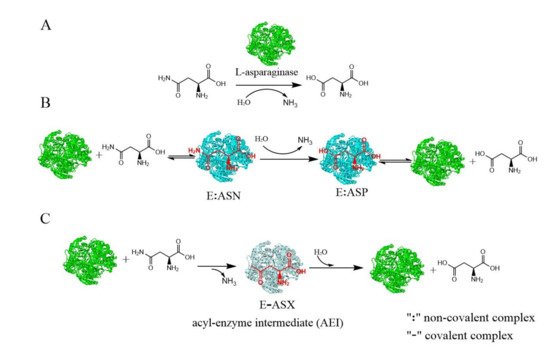
L-asparaginase (E.C.3.5.1.1) hydrolyzes L-asparagine to L-aspartic acid and ammonia, which has been widely applied in the pharmaceutical and food industries. Microbes have advantages for L-asparaginase production, and there are several commercially available forms of L-asparaginase, all of which are derived from microbes. Generally, L-asparaginase has an optimum pH range of 5.0–9.0 and an optimum temperature of between 30 and 60 °C. However, the optimum temperature of L-asparaginase from hyperthermophilic archaea is considerable higher (between 85 and 100 °C). The native properties of the enzymes can be enhanced by using immobilization techniques. The stability and recyclability of immobilized enzymes makes them more suitable for food applications.
- acrylamide
- L-asparaginase
- microbes
- immobilization
- food processing
- fermentation
- purification
1. Introduction
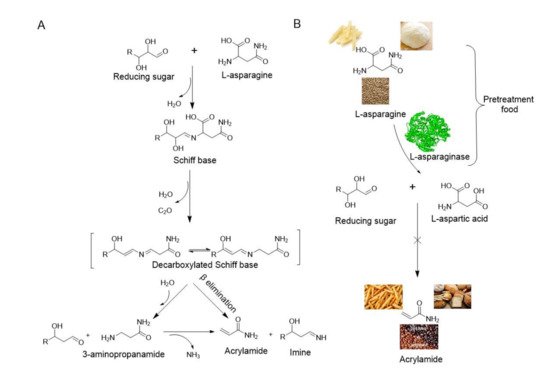
2. Application of Immobilized L-asparaginase in Food
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9081659
References
- Clementi, A. La désamidation enzymatique de l’asparagine chez les différentes espéces animales et la signification physio logique de sa presence dans l’organisme. Arch. Int. Physiol. 1922, 19, 369–398.
- Strzelczyk, P.; Zhang, D.; Dyba, M.; Wlodawer, A.; Lubkowski, J. Generalized enzymatic mechanism of catalysis by tetrameric L-asparaginases from mesophilic bacteria. Sci. Rep. 2020, 10, 17516.
- Zuo, S.; Zhang, T.; Jiang, B.; Mu, W. Recent research progress on microbial L-asparaginases. Appl. Microbiol. Biotechnol. 2015, 99, 1069–1079.
- Chand, S.; Mahajan, R.V.; Prasad, J.P.; Sahoo, D.K.; Mihooliya, K.N.; Dhar, M.S.; Sharma, G. A comprehensive review on microbial L-asparaginase: Bioprocessing, characterization, and industrial applications. Biotechnol. Appl. Biochem. 2020, 67, 619–647.
- Muneer, F.; Siddique, M.H.; Azeem, F.; Rasul, I.; Muzammil, S.; Zubair, M.; Afzal, M.; Nadeem, H. Microbial L-asparaginase: Purification, characterization and applications. Arch. Microbiol. 2020, 202, 967–981.
- Safary, A.; Moniri, R.; Hamzeh-Mivehroud, M.; Dastmalchi, S. Highly efficient novel recombinant L-asparaginase with no glutaminase activity from a new halo-thermotolerant Bacillus strain. BioImpacts BI 2019, 9, 15–23.
- Kataria, M.; Kaur, N.; Narula, R.; Kumar, K.; Kataria, S.; Verma, N. L-Asparaginase from Novel Source-Solanum nigrum and Development of Asparagine Biosensor. Pharma Innov. 2015, 4, 81.
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006.
- Svensson, K.; Abramsson, L.; Becker, W.; Glynn, A.; Hellenäs, K.E.; Lind, Y.; Rosén, J. Dietary intake of acrylamide in Sweden. Food Chem. Toxicol. 2003, 41, 1581–1586.
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450.
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449.
- Zuo, S.; Zhang, T.; Jiang, B.; Mu, W. Reduction of acrylamide level through blanching with treatment by an extremely thermostable L-asparaginase during French fries processing. Extremophiles 2015, 19, 841–851.
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526.
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide Formation Mechanism in Heated Foods. J. Agric. Food Chem. 2003, 51, 4782–4787.
- Mohan Kumar, N.S.; Shimray, C.A.; Indrani, D.; Manonmani, H.K. Reduction of Acrylamide Formation in Sweet Bread with L-Asparaginase Treatment. Food Bioprocess Technol. 2014, 7, 741–748.
- Corrêa, C.L.O.; das Merces Penha, E.; dos Anjos, M.R.; Pacheco, S.; Freitas-Silva, O.; Luna, A.S.; Gottschalk, L.M.F. Use of asparaginase for acrylamide mitigation in coffee and its influence on the content of caffeine, chlorogenic acid, and caffeic acid. Food Chem. 2021, 338, 128045.
- Lyon, F. IARC monographs on the evaluation of carcinogenic risks to humans. Some Ind. Chem. 1994, 60, 389–433.
- Commission, E. Commission Regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, 304, 24–44.
- Khalil, N.M.; Rodríguez-Couto, S.; El-Ghany, M.N.A. Characterization of Penicillium crustosum L-asparaginase and its acrylamide alleviation efficiency in roasted coffee beans at non-cytotoxic levels. Arch. Microbiol. 2021.
- Sun, Z.; Qin, R.; Li, D.; Ji, K.; Wang, T.; Cui, Z.; Huang, Y. A novel bacterial type II L-asparaginase and evaluation of its enzymatic acrylamide reduction in French fries. Int. J. Biol. Macromol. 2016, 92, 232–239.
- Kornbrust, B.A.; Stringer, M.A.; Lange, N.E.K.; Hendriksen, H.V.; Whitehurst, R.; Oort, M. Asparaginase–An enzyme for acrylamide reduction in food products. Enzym. Food Technol. 2010, 2, 59–87.
- Adebo, O.A.; Kayitesi, E.; Adebiyi, J.A.; Gbashi, S.; Temba, M.C.; Lasekan, A.; Phoku, J.Z.; Njobeh, P.B. Mitigation of acrylamide in foods: An African perspective. Acrylic Polym. Healthc. 2017, 152–172.
- Xu, F.; Oruna-Concha, M.-J.; Elmore, J.S. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016, 210, 163–171.
- Hendriksen, H.V.; Kornbrust, B.A.; Østergaard, P.R.; Stringer, M.A. Evaluating the Potential for Enzymatic Acrylamide Mitigation in a Range of Food Products Using an Asparaginase from Aspergillus oryzae. J. Agric. Food Chem. 2009, 57, 4168–4176.
- Vidya, J.; Sajitha, S.; Ushasree, M.V.; Sindhu, R.; Binod, P.; Madhavan, A.; Pandey, A. Genetic and metabolic engineering approaches for the production and delivery of L-asparaginases: An overview. Bioresour. Technol. 2017, 245, 1775–1781.
- Alam, S.; Ahmad, R.; Pranaw, K.; Mishra, P.; Khare, S.K. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour. Technol. 2018, 269, 121–126.
- Ravi, A.; Gurunathan, B. Acrylamide Mitigation in Fried Kochchi Kesel Chips Using Free and Immobilized Fungal Asparaginase. Food Technol. Biotechno.l 2018, 56, 51–57.
- Li, R.; Zhang, Z.; Pei, X.; Xia, X. Covalent Immobilization of L-Asparaginase and Optimization of Its Enzyme Reactor for Reducing Acrylamide Formation in a Heated Food Model System. Front. Bioeng. Biotechnol. 2020, 8.
