Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Obstetrics & Gynaecology
β-Thalassemia is the most prevalent single gene blood disorder, while the assessment of its susceptibility to coronavirus disease 2019 (COVID-19) warrants it a pressing biomedical priority.
- β-thalassemia
- risk
- coronavirus
1. Introduction
Identifying medical conditions with a high or potentially deadly impact on the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a critical initial step towards containment of associated morbidity and mortality risks. Given that viral stress from SARS-CoV-2 elicits anabolic responses supported by increasing blood pressure to meet enhanced oxygen needs of vital organs and organ systems, hypoxemia is rendered a high-risk medical condition [1,2]. As the most common blood disorder affecting approximately one third of the global population, anemia presents a low tolerance to hypoxemia and may have either acquired polysystemic or inherited poly- or monogenic background [3]. Monogenic anemia—which is caused by abnormal hemoglobin—is a rather prevalent medical disorder with 270 million carriers worldwide [4,5,6]. β-Thalassemia is the most common inherited single gene disorder in the world. Approximately one-third of all hemoglobinopathies and/or nearly 1.5% of the global population carry the β-thalassemia trait [7]. In this context, β-thalassemia heterozygosity is a strong candidate condition for assessing an individual’s susceptibility to COVID-19.
2. Results
Association of β-thalassemia heterozygosity with severe and critical COVID-19 symptoms
Considering the clinical spectrum of COVID-19 as a primary outcome, patients were categorized into three groups (asymptomatic and mild/ moderate/ severe and critical). No difference in chest X ray or CT scan was observed among study participants. In univariate analysis, sex (p = 0.047), age (p < 0.001), atrial fibrillation (p = 0.022), coronary disease (p = 0.041), hyperlipidemia (p = 0.014), hypertension (p < 0.001), and being heterozygous for thalassemia (p = 0.004) were associated with severe COVID-19 symptoms (Table 1). In multivariate analysis, male sex (p = 0.023), increased age (p < 0.001), and being heterozygous for thalassemia (p = 0.002) were identified as independent risk factors for severe and critical clinical COVID-19 symptoms. Specifically, males had a 1.81 times (95% CI, 1.09 to 3.01) increased possibility for severe or critical clinical symptoms; increased age was associated with increased odds of severe and clinical symptoms with OR = 1.06 (95% CI, 1.04 to 1.08). A finding of great interest is that patients who were heterozygous for thalassemia were 2.89 times (95% CI, 1.49 to 5.62) more likely to have severe and critical clinical symptoms of COVID-19 (Figure 1).
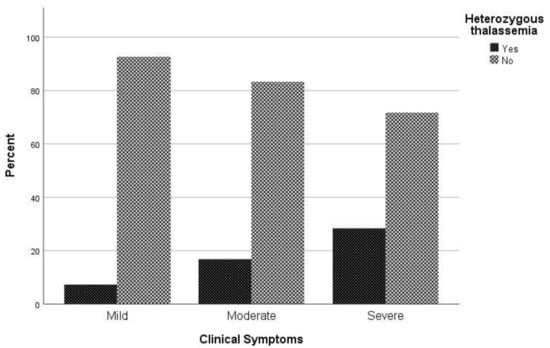
Figure 1. Proportion of β-thalassemia heterozygotes relative to non-carriers regarding clinical symptoms to COVID-19.
2.1. Association of β-Thalassemia Heterozygotes with Mortality Due to COVID-19
Regarding mortality associated with COVID-19 infection, in univariate analysis sex (p = 0.022), age (p < 0.001), atrial fibrillation (p = 0.002), respiratory disease (p = 0.027), coronary disease (p = 0.027), hypertension (p < 0.001), and being heterozygous for thalassemia (p = 0.005) were associated with mortality (Table 2). In logistic regression analysis, male patients had a 2.09 times (95% CI, 1.05 to 4.18) greater possibility of dying and patients with increased age were 1.06 times (95% CI, 1.03 to 1.09) more likely to die. It is worth noting that hyperlipidemia plays a beneficial role in COVID-19 mortality, as the odds ratio of mortality in patients with hyperlipidemia is 0.65 (95% CI 0.37–1.15). It should be highlighted that patient who are heterozygous for thalassemia have a 2.79 times (95% CI, 1.28 to 6.09) greater possibility of dying than other patients (Figure 2).
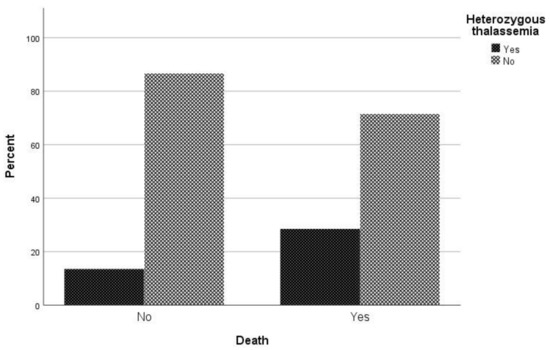
Figure 2. Proportion of β-thalassemia heterozygotes relative to non-carriers regarding mortality due to COVID-19.
2.2. Admission of COVID-19 Infected β-Thalassemia Heterozygotes to the ICU
Regarding the requirement for ICU care, it was found through univariate analysis that age (p = 0.03), respiratory disease (p = 0.043), coronary disease (p = 0.029) and hypertension (p < 0.001) were associated with ICU admission (Table 3). Through logistic regression analysis, patients with hypertension had 5.12 times (95% CI, 2.04 to 12.87) greater risk of requiring ICU care than patients without hypertension. On the contrary, hyperlipidemia was identified as a protective factor against ICU admission, with OR = 0.44 (95% CI, 0.21 to 0.94). Furthermore, in relation to the requirement for ICU care, being heterozygous for thalassemia had no effect on the possibility of admission to the ICU (p = 0.505).
2.3. Length of Hospitalization until Death
When comparing the median length of hospitalization (days) between patients being heterozygous for thalassemia and non-carriers, a statistically significant difference was observed (p = 0.046) (Figure 3). More specifically, the median duration of hospitalization among carriers and non-carriers was 12 and 17.5 days, respectively.
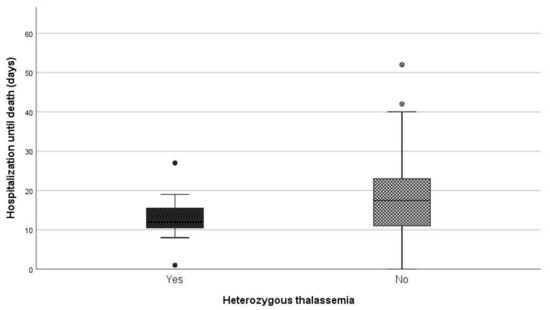
Figure 3. Days of hospitalization until death between carries and non-carriers.
2.4. Length of Hospitalization among Patients Who Survived
Regarding days of hospitalization among patients that survived COVID-19, the median duration was eight days for patients that were heterozygous for thalassemia and six days for non-carriers (p = 0.014) (Figure 4).
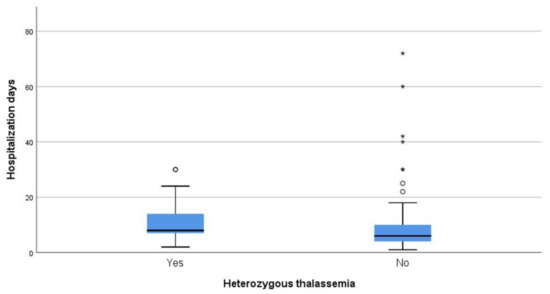
Figure 4. Days of hospitalization between carries and non-carriers that survived.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10163645
This entry is offline, you can click here to edit this entry!
