Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Chemical
Sulfide solid-state electrolytes are particularly promising for the development of high-energy all-solid-state Li metal batteries because of their high ionic conductivity and deformability. However, their unstable interface and interphase formation with electrode materials remain a great challenge.
- solid-state batteries
- solid electrolyte
- lithium metal
- interface
- interphase
- stability
1. Introduction
For decades, rechargeable lithium-ion batteries (LIBs) using organic liquid electrolytes have been commercially used as a power source for portable electronic devices [1][2][3], and their use has recently expanded to mid–large-scale electrochemical energy storage applications, including electric vehicles (EVs) and grids [4][5]. For the success of EVs, battery technology must surpass the current limitation of driving ranges by achieving a larger capacity. According to a global technical goal for 2030, the specific energy of secondary batteries must reach 500 Wh kg−1, which is much higher than the 246 Wh kg−1 of today’s typical EV batteries used in the Tesla Model 3 [6][7][8][9]. To achieve such a high energy density, a Li-metal anode is considered to be an unrivaled component, owing mainly to its extremely high theoretical specific capacity (3860 mAh g−1) and lowest reduction potential (−3.040 V vs. the standard hydrogen electrode). Safety issues have become more important than ever as battery sizes have increased. However, current LIB technology using liquid electrolytes cannot meet the requirements for the use of a Li-metal anode or safety [10][11].
In this respect, all-solid-state batteries (ASSBs), which use inorganic solid-state electrolytes (SSEs) instead of flammable organic liquid electrolytes, have emerged as promising alternatives to conventional LIBs. SSEs offer several advantages over conventional liquid electrolytes. First, ASSBs are stable even in extreme environmental conditions such as high pressure, temperature, overcharge, or external shock, and there is much less risk of ignition or explosion [12][13]. Second, it is believed that ASSBs can use a Li-metal anode since the sufficient stiffness of SSEs can mitigate the internal short circuit by suppressing the Li dendrite growth during the electrochemical cycles [14]. Third, the volumetric energy density can be improved by reducing its volume since there is no need to pack each cell and a simpler design through bipolar stacking is available for ASSBs [15][16]. Despite these advantages of ASSBs, important challenges remain to be addressed. Many SSEs display lower ionic conductivity at room temperature when compared to liquid electrolytes. Moreover, it is difficult to ensure the ion-transfer channel between the SSE and electrodes because of their unstable interfaces.
Of the various kinds of SSEs, sulfide SSEs have attracted significant attention due to their high ionic conductivity (10−2–10−4 S cm−1), which is comparable to that of organic liquid electrolytes [11][17][18]. Figure 1 summarizes the ionic conductivities of sulfide SSEs. Although the high ionic conductivity and deformability make sulfide compounds promising SSEs, several important challenges remain. Sulfide SSEs have an intrinsically narrow electrochemical stability window, which results in undesired (electro)chemical reactions between the sulfide SSE and electrodes during battery cycling [19], forming unstable interphases. Such interphase formation leads to operation failures of ASSBs. Therefore, considerable efforts are now focused on improving the interfacial stabilities between sulfide SSEs and electrode materials.
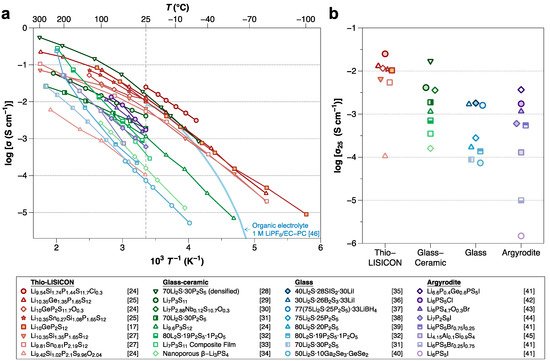
Figure 1. (a) Arrhenius-type plot of ionic conductivity for sulfide SSEs reported to date (as of February 2021). (b) The magnified view compares the conductivities at room temperature by their structure [17][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41]. Note that the light blue line is a curve from organic liquid electrolytes of 1 M LiPF6/EC-PC (50:50 vol%) for comparison [42].
In this review, we present an overview of the limitations and recent achievements in the development of sulfide SSEs, with a particular focus on the stability issue of sulfide SSEs with lithium-metal anodes and various cathode active materials. We will not discuss the conductivities of sulfide SSEs, as this topic is already well documented in previous review articles [11][43][44][45][46]. We further discuss recently proposed techniques to achieve a favorable interface between sulfide SSEs and electrodes in ASSBs. This review aims to provide not only a comprehensive description of the developments of sulfide SSEs but also insight into potential directions of future research.
2. Electrochemical Stability of Sulfide SSEs
The greatest challenge for sulfide SSEs is their very narrow electrochemical stability window, which leads to poor compatibility with electrodes. Although early works claimed that sulfide SSEs have a good electrochemical stability window of 0–4 V (vs. Li metal) based on cyclic voltammetry (CV) measurements [17][24][47], it is highly likely that these works overestimated their electrochemical stability. In the CV tests, the planar electrode geometry limits the contact area, leading to small currents, which can show kinetically extended electrochemical stability instead of a thermodynamic stability window. Later, Han et al. and Dewald et al. proved that the electrochemical stability windows of sulfide SSEs are indeed much narrower than those determined by previous CV tests [48][49]. To increase the ‘active’ contact area, they mixed Li10GeP2S12 (LGPS) thiophosphate and carbon and measured the CV of the mixture, which presented a much lower oxidation limit (<2.5 V vs. Li). In the mixture of LGPS–carbon, LGPS provides Li-transport pathways and carbon works for electron transfer. Therefore, such a configuration could show a ‘practical’ electrochemical stability window. This is critical since SSEs will encounter carbon additives in the cathode composite of ASSBs. Computational works have also demonstrated that sulfide SSEs have a narrow electrochemical stability window when compared with oxides and halides, as shown in Figure 2 [19][50][51]. Such a narrow electrochemical stability window is a major practical disadvantage of sulfide SSEs since the electrolyte must be stable over a wide range of lithium potentials between the anode chemical potential (0 eV/atom vs. Li) and the potential set by the cathode, which is near <−4.0 eV/atom for typical cathodes.
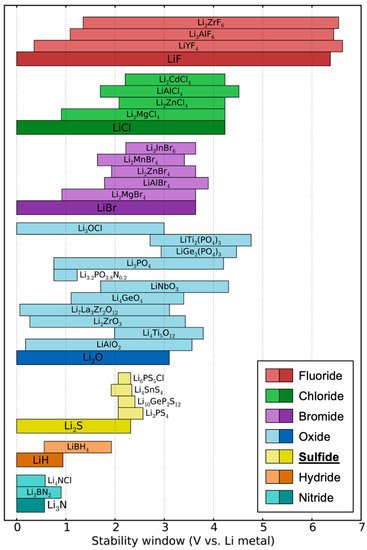
Figure 2. Theoretically driven electrochemical stability ranges of various electrolyte materials. Note that the yellow-colored sulfide electrolytes show low upper limits of less than 3.0 V. Reproduced with permission from reference [19], Copyright 2016 American Chemical Society.
This entry is adapted from the peer-reviewed paper 10.3390/electrochem2030030
References
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127.
- Nagaura, T. A lithium ion battery. In Proceedings of the 5th International Seminar on Lithium Battery Technology and Applications, Deerfield Beach, FL, USA, 5–7 March 1990.
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418.
- Bruce, P.G. Energy storage beyond the horizon: Rechargeable lithium batteries. Solid State Ion. 2008, 179, 752–760.
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854.
- U.S. DRIVE. Electrochemical Energy Storage Technical Team Roadmap; US Department of Energy: Washington, DC, USA, 2013.
- Li, C.; Negnevitsky, M.; Wang, X.; Yue, W.L.; Zou, X. Multi-criteria analysis of policies for implementing clean energy vehicles in China. Energy Policy 2019, 129, 826–840.
- Takehiko, N. The Japanese Policy and NEDO Activity for Future Mobility; Representative office in Europe, New Energy and Industrial Technology Development Organization (NEDO): Paris, France, 2017.
- Harris, S.J. Unlocking a Secret Stash of Energy. Joule 2020, 4, 1155–1157.
- Xiao, Y.; Wang, Y.; Bo, S.-H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 2020, 5, 105–126.
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669.
- Appetecchi, G.B.; Shin, J.H.; Alessandrini, F.; Passerini, S. 0.6Ah Li/V2O5 battery prototypes based on solvent-free PEO–LiN(SO2CF2CF3)2 polymer electrolytes. J. Power Sources 2005, 143, 236–242.
- Seino, Y.; Takada, K.; Kim, B.-C.; Zhang, L.; Ohta, N.; Wada, H.; Osada, M.; Sasaki, T. Synthesis of phosphorous sulfide solid electrolyte and all-solid-state lithium batteries with graphite electrode. Solid State Ion. 2005, 176, 2389–2393.
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404.
- Baba, M.; Kumagai, N.; Fujita, H.; Ohta, K.; Nishidate, K.; Komaba, S.; Kaplan, B.; Groult, H.; Devilliers, D. Multi-layered Li-ion rechargeable batteries for a high-voltage and high-current solid-state power source. J. Power Sources 2003, 119, 914–917.
- Sato, T.; Morinaga, T.; Marukane, S.; Narutomi, T.; Igarashi, T.; Kawano, Y.; Ohno, K.; Fukuda, T.; Tsujii, Y. Novel Solid-State Polymer Electrolyte of Colloidal Crystal Decorated with Ionic-Liquid Polymer Brush. Adv. Mater. 2011, 23, 4868–4872.
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686.
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries. J. Asian Ceram. Soc. 2013, 1, 17–25.
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface Stability in Solid-State Batteries. Chem. Mater. 2016, 28, 266–273.
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030.
- Kwon, O.; Hirayama, M.; Suzuki, K.; Kato, Y.; Saito, T.; Yonemura, M.; Kamiyama, T.; Kanno, R. Synthesis, structure, and conduction mechanism of the lithium superionic conductor Li10+δGe1+δP2−δS12. J. Mater. Chem. A 2014, 3, 438–446.
- Sun, Y.; Suzuki, K.; Hori, S.; Hirayama, M.; Kanno, R. Superionic Conductors: Li10+δ[SnySi1−y]1+δP2−δS12 with a Li10GeP2S12-type Structure in the Li3PS4-Li4SnS4-Li4SiS4 Quasi-ternary System. Chem. Mater. 2017, 29, 5858–5864.
- Hori, S.; Suzuki, K.; Hirayama, M.; Kato, Y.; Saito, T.; Yonemura, M.; Kanno, R. Synthesis, structure, and ionic conductivity of solid solution, Li10+δM1+δP2−δS12 (M = Si, Sn). Faraday Discuss. 2014, 176, 83–94.
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2013, 7, 627–631.
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. New, Highly Ion-Conductive Crystals Precipitated from Li2S–P2S5 Glasses. Adv. Mater. 2005, 17, 918–921.
- Homann, G.; Meister, P.; Stolz, L.; Brinkmann, J.P.; Kulisch, J.; Adermann, T.; Winter, M.; Kasnatscheew, J. High-Voltage All-Solid-State Lithium Battery with Sulfide-Based Electrolyte: Challenges for the Construction of a Bipolar Multicell Stack and How to Overcome Them. ACS Appl. Energy Mater. 2020, 3, 3162–3168.
- Minami, K.; Mizuno, F.; Hayashi, A.; Tatsumisago, M. Lithium ion conductivity of the Li2S–P2S5 glass-based electrolytes prepared by the melt quenching method. Solid State Ion. 2007, 178, 837–841.
- Mizuno, F.; Ohtomo, T.; Hayashi, A.; Tadanaga, K.; Minami, T.; Tatsumisago, M. Structure and Ionic Conductivity of Li2S-P2S5-P2O5 Glasses and Glass-Ceramics Prepared by Mechanical Milling. J. Ceram. Soc. Jpn. Suppl. 2004, 112, S709–S712.
- Tan, D.H.S.; Banerjee, A.; Deng, Z.; Wu, E.A.; Nguyen, H.; Doux, J.-M.; Wang, X.; Cheng, J.-h.; Ong, S.P.; Meng, Y.S.; et al. Enabling Thin and Flexible Solid-State Composite Electrolytes by the Scalable Solution Process. ACS Appl. Energy Mater. 2019, 2, 6542–6550.
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous High Ionic Conductivity of Nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978.
- Kennedy, J.H.; Zhang, Z.; Eckert, H. Ionically conductive sulfide-based lithium glasses. J. Non-Cryst. Solids 1990, 123, 328–338.
- Wada, H.; Menetrier, M.; Levasseur, A.; Hagenmuller, P. Preparation and ionic conductivity of new B2S3-Li2S-LiI glasses. Mater. Res. Bull. 1983, 18, 189–193.
- Yamauchi, A.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation and ionic conductivities of (100 − x)(0.75Li2S·0.25P2S5)·xLiBH4 glass electrolytes. J. Power Sources 2013, 244, 707–710.
- Dietrich, C.; Weber, D.A.; Sedlmaier, S.J.; Indris, S.; Culver, S.P.; Walter, D.; Janek, J.; Zeier, W.G. Lithium ion conductivity in Li2S–P2S5 glasses—Building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7. J. Mater. Chem. A 2017, 5, 18111–18119.
- Hayashi, A.; Hama, S.; Minami, T.; Tatsumisago, M. Formation of superionic crystals from mechanically milled Li2S–P2S5 glasses. Electrochem. Commun. 2003, 5, 111–114.
- Marple, M.A.T.; Aitken, B.G.; Kim, S.; Sen, S. Fast Li-Ion Dynamics in Stoichiometric Li2S–Ga2Se3–GeSe2 Glasses. Chem. Mater. 2017, 29, 8704–8710.
- Ohno, S.; Bernges, T.; Buchheim, J.; Duchardt, M.; Hatz, A.-K.; Kraft, M.A.; Kwak, H.; Santhosha, A.L.; Liu, Z.; Minafra, N.; et al. How Certain Are the Reported Ionic Conductivities of Thiophosphate-Based Solid Electrolytes? An Interlaboratory Study. ACS Energy Lett. 2020, 5, 910–915.
- Zhang, Z.; Zhang, L.; Liu, Y.; Yu, C.; Yan, X.; Xu, B.; Wang, L.-M. Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries. J. Alloy. Compd. 2018, 747, 227–235.
- Zhang, Z.; Zhang, L.; Yan, X.; Wang, H.; Liu, Y.; Yu, C.; Cao, X.; Eijck, L.V.; Wen, B. All-in-one improvement toward Li6PS5Br-Based solid electrolytes triggered by compositional tune. J. Power Sources 2019, 410, 162–170.
- Rangasamy, E.; Liu, Z.; Gobet, M.; Pilar, K.; Sahu, G.; Zhou, W.; Wu, H.; Greenbaum, S.; Liang, C. An iodide-based Li7P2S8I superionic conductor. J. Am. Chem. Soc. 2015, 137, 1384–1387.
- Huang, W.; Yoshino, K.; Hori, S.; Suzuki, K.; Yonemura, M.; Hirayama, M.; Kanno, R. Superionic Lithium Conductor with a Cubic Argyrodite-type Structure in the Li–Al–Si–S system. J. Solid State Chem. 2018, 270, 487–492.
- Stallworth, P.E.; Fontanella, J.J.; Wintersgill, M.C.; Scheidler, C.D.; Immel, J.J.; Greenbaum, S.G.; Gozdz, A.S. NMR, DSC and high pressure electrical conductivity studies of liquid and hybrid electrolytes. J. Power Sources 1999, 81, 739–747.
- Ma, Z.; Xue, H.-G.; Guo, S.-P. Recent achievements on sulfide-type solid electrolytes: Crystal structures and electrochemical performance. J. Mater. Sci. 2018, 53, 3927–3938.
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide Solid Electrolytes for All-Solid-State Lithium Batteries: Structure, Conductivity, Stability and Application. Energy Storage Mater. 2018, 14, 58–74.
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606.
- Kudu, Ö.U.; Famprikis, T.; Fleutot, B.; Braida, M.-D.; Mercier, T.L.; Islam, M.S.; Masquelier, C. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S−P2S5 binary system. J. Power Sources 2018, 407, 31–43.
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2S-GeS2-P2S5 System. J. Electrochem. Soc. 2001, 148, A742.
- Han, F.; Zhu, Y.; He, X.; Mo, Y.; Wang, C. Electrochemical Stability of Li10GeP2S12 and Li7La3Zr2O12 Solid Electrolytes. Adv. Energy Mater. 2016, 6, 1501590.
- Dewald, G.F.; Ohno, S.; Kraft, M.A.; Koerver, R.; Till, P.; Vargas-Barbosa, N.M.; Janek, J.; Zeier, W.G. Experimental Assessment of the Practical Oxidative Stability of Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2019, 31, 8328–8337.
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693.
- Zhu, Y.; He, X.; Mo, Y. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 2016, 4, 3253–3266.
This entry is offline, you can click here to edit this entry!
