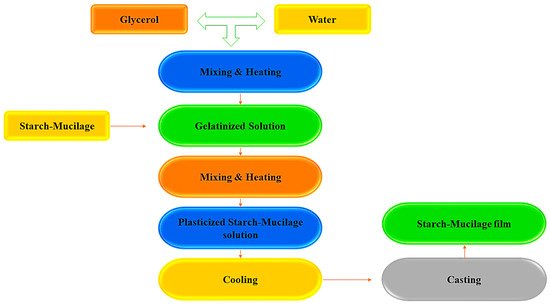
Generally, biopolymer (starch–mucilage)-based edible film can be synthesized by two methods: the casting and extrusion techniques, also known as wet and dry methods [
18]. Moreover, the solubility of starch–mucilage and other additives is an important factor for the casting method of film formation, while thermo-plasticity of starch–mucilage along with gelatinization characteristics, glass transition, and phase transitions can be recognized for the extrusion method [
19]. Among these two methods, the casting method is widely used for the synthesis of starch–mucilage films due to its low production cost and is also known as the solvent-casting method. Furthermore, the solvent casting method comprises three successive steps to prepare a film from the binary polymers (starch and mucilage): (i) solubilization of starch–mucilage sample in an appropriate solvent, (ii) casting or forming of the prepared starch–mucilage solution in molds, and (iii) drying of starch–mucilage-casted solution [
20,
21]. The synthesis of the starch–mucilage film is explained in
Figure 1.
Glycerol is most commonly used to dissolve or disperse the starch and mucilage polymers; this process is known as solubilization. The resulting solution is poured into a specified mold or glass plate during the casting process. The drying process allows the solvent to evaporate, resulting in a starch–mucilage film that binds to the mold [
22]. For the casting of films, drying techniques such as vacuum driers, microwaves, tray dryers, and hot air ovens are used to evaporate the solvents and peel the film [
23]. In this context, films were prepared from the
Plantago psyllium starch and seed mucilage by Krystyjan et al. [
24]. In their study, they proved that films prepared with binary polymers have strong physicochemical properties. Additionally, the binary combination of starch and mucilage showed high thermal stability and reduced the decomposition process as compared to film prepared by single polymer starch only. Moreover, the amount of pseudo-plasticity was decreased with the increase in the starch concentration due to galactomannans structure being composed of a mannose backbone, which interchanges with galactose chains. The breakdown of the network induced by shear forces was faster than the recovery of the structure in systems where thixotropy characteristics were dominant. Weak physical bonds were broken as a result of shear forces, and the interior structure disintegrated into separate particles. Additionally, with the addition of mucilage, the rheological behavior of film-forming solution showed various changes such as increased shear stress, higher consistency coefficient, and influence on the value of the area of the hysteresis loop [
17].
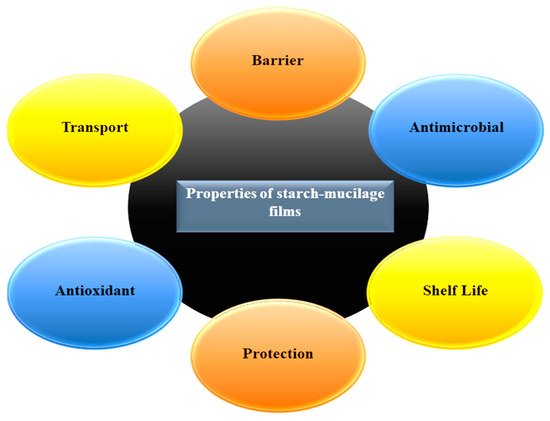
Consequently, plasticizers and cohesive matrix can be used for the development of easily peeled starch–mucilage edible films with an excellent uniform microstructure, thermal stability, barrier properties, and mechanical properties. The starch–mucilage pre-fabricated film can be used in several industrial applications, mainly in food applications. Here, the film can act as a good barrier, protector, and reduce the loss of water from food products [
21]. Therefore, they can enhance the shelf-life of food products. The thickness of the film can be decreased by increasing the concentration of starch nanocrystal due to the excellent composite formation between starch nanocrystals and mucilage [
3]. In addition, the thermal property, mechanical strength, and barrier property of starch–mucilage edible film can be improved with the increasing concentration of plasticizers. However, the casting method requires more drying time, which is the main drawback of this method. Likewise, edible films prepared from the potato husk starch and prickly pear peel Mucilage showed a positive impact on their properties such as excellent flexibility, transparency, and bright appearance. The water solubility of the film was influenced by the potato husk starch content and a high amount of glycerine and prickly pear peel leads to films with higher water retention capacity, moisture, and thickness [
25]. Therefore, the good rheological property of starch–mucilage film is highly dependent on the synthesis method of films. However, this rheological property can be controlled by adding mucilage and starch in various concentrations. Meanwhile, extrusion or dry method of film formation is usually used at a large commercial scale. This method can improve the functional or physicochemical properties and chemical structure of starch–mucilage films [
26]. Generally, this method is divided into three zones: the beginning part of the machine (feeding zone), mixing of the sample (kneading zone), and ending part from the machine (heating zone). In this regard, tubular films were prepared from
Opuntia ficus-indica mucilage (10%
W/
W) with waxy maize and acetylated or normal rice starches (70%
W/
W) and glycerol (20%
W/
W). addition of
Opuntia ficus-indica mucilage improved the functional and mechanical properties of tubular and extruded films [
16]. Because of the interaction of mucilage and glycerol as a plasticizer and the partial breakdown of the starch structure during the extrusion process, it is less susceptible to acetylation. Moreover, polyvinyl alcohol (PVOH) is an important synthetic biodegradable polymer having excellent flexibility, tear, high strength, and gas barrier properties, although it has poor dimensional stability due to high moisture absorption [
27]. Furthermore, as compared to other commercial polymers, it has a very high price. As a result, polysaccharides such as starch may be blended with renewable and abundant agro-resources to minimize production costs. Several studies have been reported to improve the compatibility of starch and polyvinyl alcohol such as fillers, cross-linking agents, compatibilizers, and plasticizers [
28]. However, as most of these cross-linking agents are usually toxic, their potential use as biomaterials has been limited. To overcome these drawbacks, the mechanical properties and water resistance of starch and polyvinyl alcohol films must be improved using nontoxic functional additives and simple modification techniques. Comparative research was executed by Gomez-Aldapa et al. [
29], and films were produced from potato starch (5%
W/
V) blended with polyvinyl alcohol (PVOH) (4%
W/
V). Therein, glycerol (25%
W/
W) was used as a plasticizer. The result of a study proved that films prepared blended with starch and PVOH are highly suitable and manageable for food packaging applications. This is because the incorporation of PVOH into potato starch enhanced the water absorption capacity, and improved mechanical and functional properties and gas permeability of the film. However, biodegradable films produced from potato starch and PVOH blends had a homogeneous appearance, with no obvious bubbles or phase separation, were transparent, and were easy to unmold. Satisfactory compatibility was observed during the processing in all the blended formulations. Moreover, bindings of hydrogen bonds between polysaccharide chains of starch–mucilage and glycerol were analyzed by Fourier-transform infrared spectroscopy (FTIR) analysis of films prepared from the potato husk starch and pear peel mucilage [
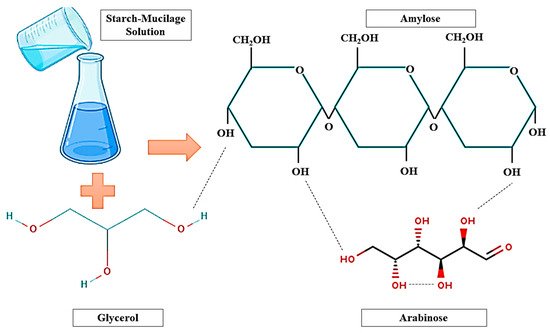
25]. Hydrogen bonding interaction (
Figure 2) is responsible for the interactions of biopolymers (starch–mucilage) with absorbed water molecules. However, mucilage consists of galacturonic acid, L-rhamnose, D-galactose, D-xylose, and L-arabinose. These carbohydrate molecules of mucilage have excellent potential to interact with other molecules such as starch (Amylose and Amylopectin) and glycerol due to their high foaming ability [
30]. Furthermore, the alternative galactose or arabinose branches prevent intramolecular hydrogen bondings from forming, also maintaining the molecule in an extended state where it may interact with the amylose molecule in the system via non-covalent hydrogen bonds, resulting in a more extended conformation [
31,
32]. As a result, the degree of pseudo-plasticity can be increased. The creation of polymer complexes encouraged by the release of amylose and low molecular weight amylopectin during the processing may be responsible for the increase in the viscosity of the starch–polysaccharide system on cooling. Moreover, the viscosity may be increased with increasing sucrose concentration due to crosslinking between starch chain and sugar units [
17].