Milk has been shown to contain a specific fraction of extracellular particles that are reported to resist digestion and are purposefully packaged with lipids, proteins, and nucleic acids to exert specific biological effects. These findings suggest that these particles may have a role in the quality of infant nutrition, particularly in the early phase of life when many of the foundations of an infant’s potential for health and overall wellness are established. However, much of the current research focuses on human or cow milk only, and there is a knowledge gap in how milk from other species, which may be more commonly consumed in different regions, could also have these reported biological effects.
- milk
- extracellular vesicle
- exosome
- ruminant
- MISEV
1. Introduction
Milk is the only food that has evolved to meet the nutritional needs of newborns, supporting growth and development while also being a significant source of nutrients in adults [1][2][3]. The domestication of livestock was a pivotal step in the consumption of non-human milk which has become a substantial source of essential nutrients in many diets globally [4][5][6]. To meet this demand, the production of milk increased from 708 million tonnes in 2009 to 883 million tonnes in 2019, with cow and buffalo milk accounting for 81% and 15% of production, respectively ( Supplementary Table S1 ) [7].
In early life, major milk components such as lactose (energy source), minerals (musculoskeletal development), and high-value biological proteins provide essential nutrition [8][9]. Milk consumption throughout life can also address malnutrition and can represent a significant proportion of overall nutrient intake in developing nations [10].
While the milk macro- and micronutrient composition is largely well established, there is considerable interest in milk-derived extracellular vesicles (EVs) and their cargoes as a source of nutrients in the classical sense, such as nucleosides, and amino acids, or as a nutritional component that influences biological functions by regulating biochemical pathways and/or interactions with the host’s gut microbiome [11][12][13][14][15][16][17][18]. Evolutionary theory suggests that milk-derived EVs and their cargoes must have a biological purpose to justify the metabolic cost required to produce them during lactation.
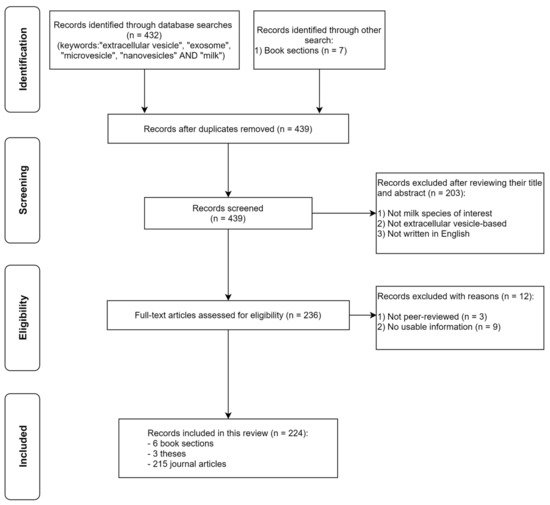
Our review covers the following: a summary of the nutritional composition of the types of milk that have been used to study milk-derived EVs, the nature and composition of these vesicles and their cargoes, the evidence for their stability and uptake in the gastrointestinal tract, their reported biological effects, and some of the key challenges in using them for studies. Methods used to identify peer-reviewed studies are shown in Figure 1 . Our review excludes any studies on plant-derived milk alternatives.
2. Milk-Derived EVs
Characterisation of isolated EVs still largely uses immunochemical (e.g., ELISA, Western blot), MS-based, and optical (e.g., nanoparticle tracking analysis (NTA), microscopy, and flow cytometry) methods. However, any of the single aforesaid detection approaches may not be sufficient to address the issues of specificity, efficiency, and consistency in EV detection. More often, multiple detection approaches are employed within the research community when it comes to EV characterisation. The progression in analytical sciences has pushed for the development of new and innovative instruments to meet the abovementioned challenges. Recent review articles have summarised the emerging new technologies available that are specifically developed for EV characterisation [19][20][21][22][23].
There are particular challenges in isolating lipids from milk-derived EVs due to the co-isolation of milk lipids (i.e., MFGs), and in milk EV isolates with a high triacylglycerol content (TAG), since MFGs contain a greater amount of TAGs in their core than EVs [24][25], potentially interfering with accurate EV lipidomic studies. In this review, MFG lipidomic studies are not included because the biophysical properties (tri-layered membrane) and cargoes of MFGs are distinctly different from those of EVs.
Major findings of nucleic acid studies conducted on EVs of different mammalian milk used to characterise the RNA composition of milk-derived EVs and exosomes.
The terminology used is based on the reference cited, and this division reflects older thinking and it is a “pool” of EVs that are responsible for the effects (circRNA, circular RNA; lncRNA, long non-coding RNA; miRNA, micro-RNA; NGS, next-generation sequencing; qPCR, quantitative PCR).
3. Stability and Uptake of Milk-Derived EVs
The studies reported in the previous sections described how EVs contain a range of lipids, proteins, and nucleic acids. The stability, i.e., resistance to degradation due to processing or digestion, of milk EVs and cargoes, and how they are taken up by the recipient cells have been studied in cows [26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42], humans [43][44][45][46][47][48], goats [49], and buffaloes [50]. These studies are summarised in Table 1 .
| Species | Findings | Ref. |
|---|---|---|
| Extracellular vesicle | ||
| Human | Stability and uptake of natural and synthetic EVs loaded with locked nucleic acid anti-sense oligonucleotides in vitro (PHH, NCI-H460 cell line, and hPSC) and in vivo (mice). | [47] |
| Cow | The impact of industrial processing on milk EVs’ structural integrity and molecular composition. | [51] |
| Cow | Cellular internalisation of EVs in vitro (hPAEC and NRCM). | [52] |
| Cow | Development of non-invasive fluorescent labelling of EVs in vitro (Caco-2 cell line), demonstrating internalisation and co-localisation of labelled EVs. | [53] |
| Cow | Time-dependent uptake of colostral miRNA, EV proteins, and isomiRs after feeding in vivo (calves). | [54] |
| Cow | Demonstrated that microwaving, but not autoclaving, agitation, or freezing, reduced miR-220c abundance. | [34] |
| Exosome | ||
| Human | Resistance of exosomes isolated from preterm human milk to in vitro digestion and internalisation in vitro (HIEC). | [28] |
| Human | Exosomal protein markers resist degradation by in vitro digestion, pH 4.5, and the uptake of digested and undigested exosomes, based on immunofluorescence imaging of exosomal protein markers in vitro (HIEC). | [44] |
| Human | Resistance of miRNA to degradation caused by incubation at 26 °C over 24 h, six freeze–thaw cycles at −20 °C, treatment with RNase A and RNase T1, and incubation at 100 °C for 10 min. | [45] |
| Human | Demonstrated the uptake of RNA ex vivo (macrophages). | [48] |
| Human + Cow | Storage at 4 °C substantially reduced the exosome content, especially miRNA, of human milk over time, and the infant formulae tested had no detectable miRNA. | [43] |
| Cow | Assessed the accumulation and effects of milk exosomes and miRNA cargoes on embryo development in C57BL/6 mice. | [55] |
| Cow | Resistance of lncRNA to degradation by in vitro digestion. | [29] |
| Cow | Resistance of paclitaxel (chemotherapeutic), encapsulated in these exosomes, to degradation and loss of efficacy from long-term storage at −80 °C for 4 weeks. | [40] |
| Cow | Resistance of 5 miRNAs to degradation by an in vitro digestion method and in vitro internalisation of exosomes. | [26] |
| Cow | Uptake of exosomes and exosome-encapsulated siRNA (both digested and undigested) in vitro (Caco-2 cell line). | [35] |
| Cow | Fermentation of milk exosomes with probiotic Streptococcus thermophiles, Lactobacilli, and Bifidobacteria reduces miR-29b and miR-21 abundance and total protein concentration. | [31] |
| Cow | Challenged the findings from a previous study [27] regarding the dietary transfer of cow milk-derived miRNA in humans. | [41] |
| Cow | Demonstrated that miR-223 and miR-125b persist in high abundance after simulated in vitro digestion (TNO TIM-1 model). Authors found that exosomes may not be the only carrier of these miRNAs in milk. | [38] |
| Cow | Uptake and bioavailability of fluorescent-labelled exosomes and their miRNA cargoes via endocytosis in vivo (C57BL/6 mice) and in vitro (HUVEC). | [37] |
| Cow | Resistance of native miRNA and anticancer compounds encapsulated in these exosomes to degradation from long-term storage at −80 °C for 6 months. | [39] |
| Cow | Uptake of miRNA in differentiated and undifferentiated THP-1 cells. | [30] |
| Cow | Uptake and transport of miRNA by endocytosis in vitro (Caco-2 and IEC-6 cell lines). | [36] |
| Cow | Uptake of miR-29b and miR-200c in a randomised crossover feeding study, in C57BL/6J mice (± miRNA depletion), and human peripheral blood mononuclear cells (PBMCs). | [27] |
| Cow + Pig + Mice | Cross-species biodistribution profile of miRNAs in mice and pig model. | [56] |
| Goat + cancer cell lines | A novel approach of covalently labelled exosomes with commercial fluorophores in vitro (U87 and B16F10 cell lines) and in vivo (C57BL/6 mice). | [57] |
| Goat | Uptake, bioavailability, and tissue distribution of radiolabelled (reduced technetium, 99mTc (IV)) exosomes using non-invasive single-photon emission computed tomography imaging in Balb/C mice. | [49] |
| Microvesicles/Nanovesicles/Other | ||
| Human | Presence of immune-related miRNA in human milk, two of which were present in exosomes. miR-21 and miR-181a were resistant to degradation by RNase, pH 1, and freeze–thaw, indicating an extracellular protective mechanism. | [46] |
| Cow | Pasteurisation and homogenisation, but not 4 °C storage, substantially reduce the abundance of miR-200c and miR29b in four types of milk tested. Somatic cells in the milk accounted for <1% of the abundance of these miRNAs in milk, consistent with these miRNAs packaged in extracellular structures such as EVs. | [42] |
| Cow | Presence of mRNA and miRNA which were resistant to degradation by RNase, pH 2, incubation at 37 °C, but not Triton X-100, indicating an extracellular protective mechanism. | [33] |
| Cow | Presence of mRNA and miRNA in both samples. These RNAs were resistant to degradation by pH 2, indicating an extracellular protective mechanism. | [32] |
| Buffalo | Demonstrated that 4 °C storage and multiple freeze–thaws reduced the abundance of miR-21 and miR-500. | [50] |
These studies show that the structure of EVs protects them against harsh conditions, such as low pH, temperature variations, or high concentrations of RNase. This capacity to resist degradation and digestion underpins the study of their potential biological effects, either as nutrient delivery or as drug delivery vehicles.
4. Biological Effects of Milk-Derived EVs
The previous sections indicate that milk-derived EVs may contain bioactive components, which are protected against degradation and digestion. The studies to date that have focused on the biological effects of milk-derived EVs are highlighted in Table 7. The majority of these studies used human or cow milk EVs, and there is a clear knowledge gap regarding whether milk from other species has similar or different effects.
The studies researched the effects of EVs and exosomes on the gut microbiota [31][56][58], on the use of a delivery vehicle [59][26][60][35][40][61][62][63][64][65][66], in the immune response [67][30][68][69][48][70][71][72][73][74][75], in diseases such as cancer, [76][77][36][63][66][74][78][79][80][81][82][83][84][85][86][87], and in other aspects of cell biology [88][89][90][91][92][93][94][95]. These studies show that milk-derived EVs can have meaningful biological effects in the model systems used, forming a basis for future research.
Major findings of studies on the biological effects of mammalian milk-derived EVs.
The terminology used is based on the reference cited, and this division reflects older thinking and is a “pool” of EVs that are responsible for the effects (ASC, adipose-derived stem cell; DSS, dextran sulphate sodium; hPAEC: human pulmonary artery endothelial cell; MDC, monocyte-derived dendritic cell; MSC, mesenchymal stem cell; NEC, necrotising enterocolitis; NRCM, neonatal rate cardiac myocyte; PBMC, peripheral blood mononuclear cell; SCFA, short-chain fatty acid; LPS, lipopolysaccharide).
This entry is adapted from the peer-reviewed paper 10.3390/nu13082505
References
- Silva, A.R.; Silva, M.M.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972.
- Moatsou, G.; Sakkas, L. Sheep milk components: Focus on nutritional advantages and biofunctional potential. Small Rumin. Res. 2019, 180, 86–99.
- Oftedal, O.T. The evolution of lactation in mammalian species. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 1–10.
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92.
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302.
- Evershed, R.P.; Payne, S.; Sherratt, A.G.; Copley, M.S.; Coolidge, J.; Urem-Kotsu, D.; Kotsakis, K.; Özdoğan, M.; Özdoğan, A.E.; Nieuwenhuyse, O.; et al. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature 2008, 455, 528–531.
- FAOSTAT. Livestock Primary (Total World Milk Production Quantity, 2009 and 2019). Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 7 April 2021).
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2019, 59, 19–34.
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2011, 92, 445–474.
- Muehlhoff, E.; Bennett, A.; McMahon, D. Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013.
- Galley, J.D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745.
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11.
- Melnik, B.C.; Schmitz, G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019, 17, 1–33.
- Munir, J.; Lee, M.; Ryu, S. Exosomes in Food: Health Benefits and Clinical Relevance in Diseases. Adv. Nutr. 2019, 11, 687–696.
- Zempleni, J. Milk exosomes: Beyond dietary microRNAs. Genes Nutr. 2017, 12, 1–4.
- Gomez, C.D.L.T.; Goreham, R.V.; Bech-Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92.
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361.
- Foster, B.P.; Balassa, T.; Benen, T.D.; Dominovic, M.; Elmadjian, G.K.; Florova, V.; Fransolet, M.D.; Kestlerova, A.; Kmiecik, G.; Kostadinova, I.A.; et al. Extracellular vesicles in blood, milk and body fluids of the female and male urogenital tract and with special regard to reproduction. Crit. Rev. Clin. Lab. Sci. 2016, 53, 379–395.
- Boriachek, K.; Islam, N.; Möller, A.; Salomon, C.; Nguyen, N.-T.; Hossain, S.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2017, 14.
- Vogel, R.; Savage, J.; Muzard, J.; Della Camera, G.; Vella, G.; Law, A.; Marchioni, M.; Mehn, D.; Geiss, O.; Peacock, B.; et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? J. Extracell. Vesicles 2021, 10, e12052.
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950.
- Shpacovitch, V.; Hergenröder, R. Optical and surface plasmonic approaches to characterize extracellular vesicles: A review. Anal. Chim. Acta 2018, 1005, 1–15.
- Chia, B.S.; Low, Y.P.; Wang, Q.; Li, P.; Gao, Z. Advances in exosome quantification techniques. TrAC Trends Anal. Chem. 2017, 86, 93–106.
- Bernard, L.; Bonnet, M.; Delavaud, C.; Delosière, M.; Ferlay, A.; Fougère, H.; Graulet, B. Milk Fat Globule in Ruminant: Major and Minor Compounds, Nutritional Regulation and Differences Among Species. Eur. J. Lipid Sci. Technol. 2018, 120, 1700039.
- Blans, K.; Hansen, M.S.; Sørensen, L.V.; Hvam, M.L.; Howard, K.A.; Moeller, A.; Wiking, L.; Larsen, L.B.; Rasmussen, J.T. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2017, 6, 1294340.
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. J. Funct. Foods 2017, 34, 431–439.
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs Are Absorbed in Biologically Meaningful Amounts from Nutritionally Relevant Doses of Cow Milk and Affect Gene Expression in Peripheral Blood Mononuclear Cells, HEK-293 Kidney Cell Cultures, and Mouse Livers. J. Nutr. 2014, 144, 1495–1500.
- Kahn, S.; Liao, Y.; Du, X.; Xu, W.; Li, J.; Lönnerdal, B. Exosomal MicroRNAs in Milk from Mothers Delivering Preterm Infants Survive In Vitro Digestion and Are Taken Up by Human Intestinal Cells. Mol. Nutr. Food Res. 2018, 62, e1701050.
- Zeng, B.; Chen, T.; Xie, M.-Y.; Luo, J.-Y.; He, J.-J.; Xi, Q.-Y.; Sun, J.-J.; Zhang, Y.-L. Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. J. Dairy Sci. 2019, 102, 6726–6737.
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933.
- Yu, S.; Zhao, Z.-H.; Sun, L.; Li, P. Fermentation Results in Quantitative Changes in Milk-Derived Exosomes and Different Effects on Cell Growth and Survival. J. Agric. Food Chem. 2017, 65, 1220–1228.
- Hata, T.; Murakami, K.; Nakatani, H.; Yamamoto, Y.; Matsuda, T.; Aoki, N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 2010, 396, 528–533.
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012, 95, 4831–4841.
- Zhao, Z.; Yu, S.; Xu, M.; Li, P. Effects of microwave on extracellular vesicles and microRNA in milk. J. Dairy Sci. 2018, 101, 2932–2940.
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Small Interfering RNA in Milk Exosomes Is Resistant to Digestion and Crosses the Intestinal Barrier In Vitro. J. Agric. Food Chem. 2017, 65, 9506–9513.
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206.
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am. J. Physiol. Cell Physiol. 2016, 310, C800–C807.
- Benmoussa, A.; Lee, C.H.C.; Laffont, B.; Savard, P.; Laugier, J.; Boilard, E.; Gilbert, C.; Fliss, I.; Provost, P. Commercial Dairy Cow Milk microRNAs Resist Digestion under Simulated Gastrointestinal Tract Conditions. J. Nutr. 2016, 146, 2206–2215.
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61.
- Agrawal, A.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636.
- Auerbach, A.; Vyas, G.; Li, A.; Halushka, M.; Witwer, K.W. Uptake of dietary milk miRNAs by adult humans: A validation study. F1000Research 2016, 5, 721.
- Howard, K.M.; Kusuma, R.J.; Baier, S.R.; Friemel, T.; Markham, L.; Vanamala, J.; Zempleni, A.J. Loss of miRNAs during Processing and Storage of Cow’s (Bos taurus) Milk. J. Agric. Food Chem. 2015, 63, 588–592.
- Leiferman, A.; Shu, J.; Upadhyaya, B.; Cui, J.; Zempleni, J. Storage of Extracellular Vesicles in Human Milk, and MicroRNA Profiles in Human Milk Exosomes and Infant Formulas. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 235–238.
- Liao, Y.; Du, X.; Yalin, L.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61.
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related MicroRNAs are Abundant in Breast Milk Exosomes. Int. J. Biol. Sci. 2012, 8, 118–123.
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7.
- Grossen, P.; Portmann, M.; Koller, E.; Duschmalé, M.; Minz, T.; Sewing, S.; Pandya, N.J.; van Geijtenbeek, S.K.; Ducret, A.; Kusznir, E.-A.; et al. Evaluation of bovine milk extracellular vesicles for the delivery of locked nucleic acid antisense oligonucleotides. Eur. J. Pharm. Biopharm. 2021, 158, 198–210.
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9.
- González, M.I.; Martín-Duque, P.; Desco, M.; Salinas, B. Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials 2020, 10, 1062.
- Baddela, V.S.; Nayan, V.; Rani, P.; Onteru, S.K.; Singh, D. Physicochemical Biomolecular Insights into Buffalo Milk-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2015, 178, 544–557.
- Kleinjan, M.; van Herwijnen, M.J.; Libregts, S.F.; van Neerven, R.J.; Feitsma, A.L.; Wauben, M.H. Regular Industrial Processing of Bovine Milk Impacts the Integrity and Molecular Composition of Extracellular Vesicles. J. Nutr. 2021, 151, 1416–1425.
- Bedoya, N. Impact of Bovine Milk Extracellular Vesicles and Their MicroRNA Cargoes on the Cardiovascular System; Icahn School of Medicine at Mount Sinai: New York, NY, USA, 2020.
- Hansen, M.S.; Gadegaard, I.S.E.; Arnspang, E.C.; Blans, K.; Nejsum, L.N.; Rasmussen, J.T. Specific and Non-Invasive Fluorescent Labelling of Extracellular Vesicles for Evaluation of Intracellular Processing by Intestinal Epithelial Cells. Biomedicines 2020, 8, 211.
- Kirchner, B.; Buschmann, D.; Paul, V.; Pfaffl, M.W. Postprandial transfer of colostral extracellular vesicles and their protein and miRNA cargo in neonatal calves. PLoS ONE 2020, 15, e0229606.
- Sadri, M.; Shu, J.; Kachman, S.D.; Cui, J.; Zempleni, J. Milk exosomes and miRNA cross the placenta and promote embryo survival in mice. Reproduction 2020, 160, 501–509.
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 1–11.
- González, M.; González-Arjona, M.; Santos-Coquillat, A.; Vaquero, J.; Vázquez-Ogando, E.; de Molina, A.; Peinado, H.; Desco, M.; Salinas, B. Covalently Labeled Fluorescent Exosomes for In Vitro and In Vivo Applications. Biomedicines 2021, 9, 81.
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, e1901251.
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132.
- Matsuda, A.; Moirangthem, A.; Angom, R.S.; Ishiguro, K.; Driscoll, J.; Yan, I.K.; Mukhopadhyay, D.; Patel, T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2019, 40, 706–718.
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007.
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes—Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195.
- Mudd, A.M.; Gu, T.; Munagala, R.; Jeyabalan, J.; Egilmez, N.K.; Gupta, R.C. Chemoprevention of Colorectal Cancer by Anthocyanidins and Mitigation of Metabolic Shifts Induced by Dysbiosis of the Gut Microbiome. Cancer Prev. Res. 2019, 13, 41–52.
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes from Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505.
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Natural ligand-receptor mediated loading of siRNA in milk derived exosomes. J. Biotechnol. 2020, 318, 1–9.
- Aqil, F.; Kausar, H.; Agrawal, A.; Jeyabalan, J.; Kyakulaga, A.-H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21.
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978.
- Xie, M.-Y.; Hou, L.-J.; Sun, J.-J.; Zeng, B.; Xi, Q.-Y.; Luo, J.-Y.; Chen, T.; Zhang, Y.-L. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and p53 Pathways in Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 9477–9491.
- Maburutse, B.E.; Park, M.-R.; Oh, S.; Kim, Y. Evaluation and Characterization of Milk-derived Microvescicle Isolated from Bovine Colostrum. Food Sci. Anim. Resour. 2017, 37, 654–662.
- Nordgren, T.M.; Heires, A.J.; Zempleni, J.; Swanson, B.J.; Wichman, C.; Romberger, D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019, 64, 110–120.
- Pieters, B.C.H.; Arntz, O.J.; Bennink, M.B.; Broeren, M.; van Caam, A.; Koenders, M.I.; Van Lent, P.L.E.M.; Berg, W.B.V.D.; De Vries, M.; Van Der Kraan, P.M.; et al. Commercial Cow Milk Contains Physically Stable Extracellular Vesicles Expressing Immunoregulatory TGF-β. PLoS ONE 2015, 10, e0121123.
- Maji, S.; Yan, I.K.; Parasramka, M.; Mohankumar, S.; Matsuda, A.; Patel, T. In vitrotoxicology studies of extracellular vesicles. J. Appl. Toxicol. 2016, 37, 310–318.
- Näslund, T.I.; Proulx, D.P.; Paredes, P.T.; Vallhov, H.; Sandberg, J.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180.
- Matic, S.; D’Souza, D.H.; Wu, T.; Pangloli, P.; Dia, V.P. Bovine Milk Exosomes Affect Proliferation and Protect Macrophages against Cisplatin-Induced Cytotoxicity. Immunol. Investig. 2020, 49, 711–725.
- Stremmel, W.; Weiskirchen, R.; Melnik, B.C. Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment. Inflamm. Intest. Dis. 2020, 5, 117–123.
- Benmoussa, A.; Diallo, I.; Salem, M.; Michel, S.; Gilbert, C.; Sévigny, J.; Provost, P. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci. Rep. 2019, 9, 1–16.
- Reif, S.; Shiff, Y.E.; Golan-Gerstl, R.; Reif, S.; Shiff, Y.E.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 17, 325.
- Arntz, O.J.; Pieters, B.C.; Oliveira, M.; Broeren, M.; Bennink, M.B.; De Vries, M.; Van Lent, P.L.; Koenders, M.I.; Berg, W.B.V.D.; Van Der Kraan, P.M.; et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712.
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2019, 36, 155–163.
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284.
- Reif, S.; Elbaum-Shiff, Y.; Koroukhov, N.; Shilo, I.; Musseri, M.; Golan-Gerstl, R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients 2020, 12, 2589.
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 2019, 14, e0211431.
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J. Pediatr. Surg. 2017, 52, 755–759.
- Qin, W.; Tsukasaki, Y.; Dasgupta, S.; Mukhopadhyay, N.; Ikebe, M.; Sauter, E.R. Exosomes in Human Breast Milk Promote EMT. Clin. Cancer Res. 2016, 22, 4517–4524.
- Leiferman, A.; Shu, J.; Grove, R.; Cui, J.; Adamec, J.; Zempleni, J. A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J. Nutr. Biochem. 2018, 59, 123–128.
- Mobley, C.B.; Mumford, P.; McCarthy, J.J.; Miller, M.E.; Young, K.C.; Martin, J.S.; Beck, D.T.; Lockwood, C.; Roberts, M.D. Whey protein-derived exosomes increase protein synthesis and hypertrophy in C2C12 myotubes. J. Dairy Sci. 2017, 100, 48–64.
- Paredes, P.T.; Gutzeit, C.; Johansson, S.; Admyre, C.; Stenius, F.; Alm, J.; Scheynius, A.; Gabrielsson, S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy 2014, 69, 463–471.
- Chen, T.; Xi, Q.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wang, S.-B.; Shu, G.; Wang, L.-N.; Zhu, X.-T.; et al. Revelation of mRNAs and proteins in porcine milk exosomes by transcriptomic and proteomic analysis. BMC Vet. Res. 2017, 13, 1–14.
- Lin, D.; Chen, T.; Xie, M.; Li, M.; Zeng, B.; Sun, R.; Zhu, Y.; Ye, D.; Wu, J.; Sun, J.; et al. Oral Administration of Bovine and Porcine Milk Exosome Alter miRNAs Profiles in Piglet Serum. Sci. Rep. 2020, 10, 6983.
- Oliveira, M.; Di Ceglie, I.; Arntz, O.J.; Berg, W.B.V.D.; Hoogen, F.H.J.V.D.; Ferreira, A.; Van Lent, P.L.; Van De Loo, F.A. Milk-Derived Nanoparticle Fraction Promotes the Formation of Small Osteoclasts But Reduces Bone Resorption. J. Cell Physiol. 2016, 232, 225–233.
- Oliveira, M.C.; Pieters, B.C.H.; Guimarães, P.B.; Duffles, L.F.; Heredia, J.E.; Silveira, A.L.M.; Oliveira, A.C.C.; Teixeira, M.M.; Ferreira, A.V.M.; Silva, T.A.; et al. Bovine Milk Extracellular Vesicles Are Osteoprotective by Increasing Osteocyte Numbers and Targeting RANKL/OPG System in Experimental Models of Bone Loss. Front. Bioeng. Biotechnol. 2020, 8, 891.
- Golan-Gerstl, R.; Shiff, Y.E.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61.
- Yu, S.; Zhao, Z.-H.; Xu, X.; Li, M.; Li, P. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 2019, 272, 372–378.
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integr. Cancer Ther. 2018, 17, 1235–1246.
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1–7.
