Non-cell-specific therapeutics or therapeutics designed to tackle aberrant pathways within neurons failed to slow down or halt neurodegeneration. It is therefore time to pursue alternative strategies. In line with this, astrocytes emerge as promising therapeutic targets in various neurodegenerative disorders, an hypothesis supported by their importance to maintain the central nervous system homeostasis in health conditions as well as their fundamental and multifaced role in pathological conditions.
- neurodegenerative diseases
- astrocytes
- neuroinflammation
- rehabilitation
- targeted therapy
what follows is an extract of our recent review "Challenges and Opportunities of Targeting Astrocytes to Halt Neurodegenerative Disorders" https://doi.org/10.3390/cells10082019
1. Introduction
Among glia, astrocytes emerge as a critical and highly heterogeneous population that perform a wide array of functions, spanning from maintaining water and ion homeostasis to modulating neuronal transmission (reviewed in [2,3]). In addition to these fundamental interactions with neurons, they exchange signals also with other glial cell populations and they intimately interact with the blood capillaries to maintain the blood-brain barrier (BBB, [4]) and the glymphatic system [5]. Considering the importance of astrocyte functions for CNS performance, it is reasonable to postulate that astrocyte dysfunction can be a primary event of a pathogenic cascade ultimately leading to neuronal loss. An example that corroborates this view is the case of Alexander’s disease [6], a lethal leukodystrophy caused by mutations in the glial fibrillary acidic protein (GFAP) gene, which encodes an astrocytic intermediate filament protein. In keeping with this, autoantibodies against the astrocytic water channel protein Aquaporin-4 (AQP4) were shown to cause ~80% of occurrences of neuromyelitis optica, an autoimmune condition with a neurodegenerative component [7]. Finally, recent findings suggest that neurotrophic viruses, such as Zika and West Nile viruses, but also SARS-CoV-2, harm the CNS by interfering with astrocyte functions [8]. This amount of evidence provides a first argument challenging the neuron-centric dogma. The second element of confutation of the neuron-centric hypothesis is represented by the evidence that astrocyte dysfunction can also play a key role in the pathogenesis of several chronic neurodegenerative conditions, including Alzheimer’s (AD, [9]), Parkinson’s (PD, [10]), Huntington’s diseases (HD, [11]), and Amyotrophic Lateral Sclerosis (ALS, [12]). A third indication supporting the importance of glial cells in the diseased CNS is based on the growing awareness that “reactive gliosis” cannot be simply considered a stereotyped passive “reaction” to an insult. Rather, it is a highly articulated process where microglia, peripheral immune cells, and astrocytes sense neuronal distress and exchange signals to mount a tailor-made inflammatory milieu in response to distinct forms of injuries. This phenomenon gives rise to a neuroinflammatory reaction that can have either a neuroprotective or a detrimental impact on neurons, depending on the nature and the persistency of the insult (reviewed in [13]).
2. Astrocyte Replacement Therapy
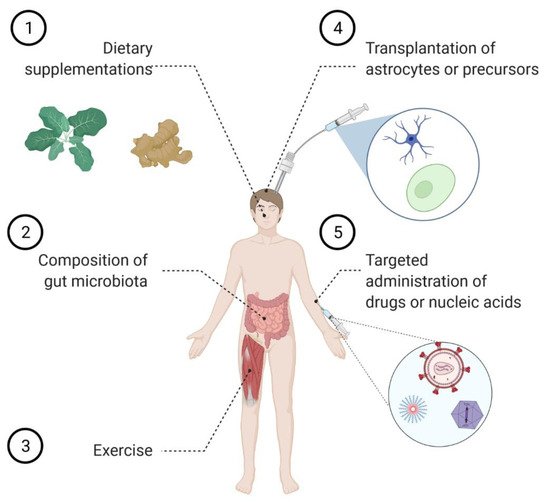
3. Modulating Astrocyte Function by Tuning the Incoming Signaling
By virtue of their strategic position within the CNS, astrocytes are particularly prone to integrate signals arising from different neural cell types as well as from the blood vessels. Because microglia-derived cytokines were reported to drive astrocyte conversion into the neurotoxic A1 phenotype, it was initially hypothesized that blocking this process could be a beneficial therapeutic approach to halt neurodegeneration.
Astrocytes modulate their functions not only by interacting with microglia-derived factors but also in response to signals arising from endothelial cells, which are major constituents of the BBB. For example, endothelial cells were reported to induce Notch signaling in astrocytes, thereby boosting the expression of the astrocyte-specific glutamate transporter EAAT2 (GLT1 in rodents) [109], a protein involved in the maintenance of extracellular glutamate concentrations below excitotoxic levels and a therapeutic target of outstanding relevance for many neurological conditions.
During neuropathology, the BBB can become impaired and lead to soluble factors and inflammatory cell infiltration into the perivascular space. Interestingly, in EAE, the cross-talk between endothelial cells and astrocytes was shown to unleash the expression of tight junction molecules in astrocytes, thus restricting the access to the parenchyma of plasmatic proteins and inflammatory cells, as well as limiting the detrimental phenotype in mice [110,111].
Ground-breaking studies published in the last few years demonstrated that astrocytes are sensitive to factors of various nature, originating also outside the CNS. In particular, circulating levels of the insulin-like growth factor-1 (IGF-1) were reported to regulate the astrocyte expression of several glutamatergic receptors and the production of eicosanoids, which ensure the optimal neurovascular coupling necessary for intense neuronal activity [113].
Finally, astrocytes have been reported to respond to pesticides and toxins. For example, the herbicide Linuron was shown to target the unfolded protein response in the astrocytes, thereby boosting their neurotoxic role in a mouse model of multiple sclerosis [131].
4. Shared Pathways of Astrocyte Dysfunction in Neurodegenerative Diseases
Several molecular mechanisms shape the response of the astrocytes to distinct neurodegenerative disorders, such as for example AD [133], ALS [12], HD [134], and PD [135]. The identification of common pathogenetic signaling cascades is full of implications, as it suggests that therapeutic agents designed to target astrocytes might have a broad spectrum of applications and be an effective add-on therapy. One of the recurring features in multiple pathologies is the loss of the astrocyte-specific glutamate transporter EAAT2, an event that might lead to neurotoxic accumulation of extracellular glutamate (reviewed in [136]). Surprisingly, the antibiotic ceftriaxone was shown to enhance EAAT2 expression [137] and to successfully ameliorate the phenotype of rodent models of different neurodegenerative conditions [138,139,140,141,142,143]. These promising findings led to the prompt activation of clinical trials to evaluate its therapeutic value in ALS (NCT00349622; NCT00718393) and PD dementia (NCT03413384). Regrettably, a Phase III clinical trial failed at demonstrating the efficacy of ceftriaxone in both slowing down disease progression and prolonging survival of ALS patients [144], while the outcome of the PD dementia trial remains to be determined because patients are still being recruited. Another small molecule apt at enhancing EAAT2 expression is LDN/OSU0212320 [145]. Interestingly, the administration of this compound successfully ameliorated the phenotype of rodent models of ALS [145], AD [146], pain [147], migraine [148], and, very recently, stroke, where the beneficial effect was observed in male, but not in female animals [149]. This last observation remarks that not only the development of neurological conditions, but also the response to treatment can be gender-dependent and calls for the design of preclinical and clinical studies taking this option into consideration.
Notably, selective Nrf2 activation in the astrocytes ameliorated the pathological phenotype in animal models of both ALS [161] and PD [162,163], while its genetic ablation worsened the development of EAE in mice [164]. Furthermore, the neuroprotective effect of transplanted astrocytes in a mouse model of PD was reported to rely on their ability to mount an Nrf2-mediated anti-oxidative and pro-survival environment [90]. At variance with this, ALS mice crossed with animals overexpressing Nrf2 in neurons [165] or subjected to neuronal-targeted viral-mediated gene therapy [166] did not exhibit an extended lifespan. Finally, systemic administration of many promising compounds, according to the in vitro studies, had moderate beneficial effects on animal models of ALS [167,168,169], PD [160,170], and AD [158]. Taken together, these discoveries support the view that Nrf2-activating compounds are indeed promising candidates to control several neurodegenerative conditions as long as the therapy is precisely targeted to the astrocytes.
NF-kB is a ubiquitously expressed transcription factor complex that plays a crucial role in the expression of various inflammatory genes. It is widely acknowledged that NF-kB is activated in the astrocytes in the context of several neurodegenerative disorders. However, a point that remains little understood is whether its activation in the astrocytes triggers a neuroprotective or a neurotoxic response. In particular, astrocyte-specific ablation of NF-kB activity was shown to be beneficial in a mouse model of PD [171], as well as in Drosophila models of Spinocerebellar ataxia 3 and AD [172]. Conversely, this approach led to conflicting results in the context of ALS. In murine models of ALS, it was initially noted that preventing NF-kB activation in astrocytes did not ameliorate the pathological phenotype [173,174] or it was even detrimental [175]. In line with these observations, we demonstrated that activating NF-kB in astrocytes can lead to the production of growth factors that are known to support neuronal survival, namely brain-derived neurotrophic factor (BDNF) and GDNF [176]. However, pharmacological treatment with the NF-kB inhibitor Withaferin A ameliorated the phenotype of several models of ALS [177,178,179].
5. Strategies to Target Astrocytes
A particularly flexible formulation to achieve delivery to the CNS is the synthesis and the assembly of nanoparticles (NPs) apt at delivering nucleic acids, proteins, and small molecules [186].
Innovative strategies are necessary to obtain delivery of chemicals specifically to astrocytes in the context of neuroinflammatory and neurodegenerative disorders, ideally upon systemic administration. To achieve these goals, research is expanding in parallel in two directions. On the one hand, NPs can be decorated with different types of peptidic moieties to achieve both the transport across the BBB and the uptake by the astrocytes. On the other hand, different types of chemistry allowed obtaining NPs apt at selectively targeting astrocytes and delivering nucleic acids [196,197,198] or small molecules [199].
5.1. Peptide-Based Delivery
5.2. Viral Delivery
Viruses have co-evolved to selectively invade host cells and take control of their machinery and metabolism to replicate, thus implying that they are natural gene delivery vectors.
This major pitfall was overcome in 2009 when Foust et al. discovered that the adeno-associated virus serotype 9 (AAV9) mainly transduces neurons when administered to neonate mice, while it infects also astrocytes in adult animals [207] and in non-human primates [208]. This promising result prompted additional investigations aiming at increasing the specificity of gene transfer to the astrocytes by either modifying the genome vector and/or by developing engineered capsids to increase the tropism towards astrocytes. In particular, cell-specific gene expression was achieved using astrocyte-specific promoters [209,210,211,212,213,214,215,216,217,218,219].
6. Astrocyte Role in Adult Neurogenesis and as Neuronal Precursors
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cells10082019
