Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Salinity is a growing problem affecting soils and agriculture in many parts of the world. The presence of salt in plant cells disrupts many basic metabolic processes, contributing to severe negative effects on plant development and growth.
- salinity stress
- photosynthesis
- chloroplast
- plastid
- osmolytes
- osmotic adjustment
1. Introduction
Soil quality in many parts of the U.S. and worldwide is susceptible to a variety of stresses, including drought, temperature, deterioration due to erosion and other factors, and increasing salinity due to evaporation and/or irrigation practices. At the same time the human population is growing and in many regions high-quality agricultural land is decreasing due to the expansion of urban areas [1].
Salinity is inhibitory to the growth and development of many plants, including most crops [2][3][4][5]. It affects all cellular processes, including disruption of cellular homeostasis, impairment of photosynthesis, mRNA processing, transcription, protein synthesis, disruption of energy metabolisms, amino acid biosynthesis as well as lipid metabolism [6][7][8][9][10]. In response to increasing salt, plant cells activate specific Na+ and Cl− ion transporters and adjust the accumulation of cytosolic K+ [10][11][12]. Plant cells must also undergo osmotic adjustment, which is accomplished in many ways, including the production of organic osmolytes such as glycine betaine, proline, some sugars, and polyamines, of which most are synthesized in the chloroplast [3][10].
Chloroplasts belong to a family of cellular organelles commonly found in plant and algal cells known as plastids. Green plastids—chloroplasts—are the site where atmospheric CO2 fixation occurs through a series of biochemical reactions called the Calvin–Benson cycle by utilizing the energy produced by the light reactions of photosynthesis [13]. Elevated salinity levels affect many cellular processes, including photosynthesis, the major function of chloroplasts. The presence of salt in the soil may cause both osmotic and ionic stresses [14], which may hinder photosynthesis through the diffusional (stomatal, mesophyll and boundary layer resistance to CO2) and/or non-diffusional (photochemical and biochemical) limitations of carbon fixation [6][15][16][17][18][19][20]. Salinity exposure is also known to decrease the chlorophyll content in many plants [21][22]. However, salt-resistant plants, particularly those with a C4 mechanism, may overcome the inhibitory effect of salinity on CO2 fixation more effectively [6][23].
In general, when plants are exposed to salt stress, the very first response is osmotic shock followed by induction of stomatal closure. Stomatal closure, in turn, limits photosynthetic capacity by the restriction of CO2 supply. However, research has shown that increasing the external CO2 concentration under salt stress did not lead to an increase in photosynthesis rates in many cases. This observation suggests the involvement of some non-stomatal components in photosynthesis reduction under salinity, such as overproduction of reactive oxygen species (ROS) and the depletion of K+ inside plant cells due to the accumulation of Na+ [24][25]. This results in the disruption of ionic homeostasis in chloroplasts.
Besides CO2 fixation, thylakoid reactions are also affected by salinity [6][18][26]. The most commonly studied parameters in this context are the maximum quantum efficiency of the PSII reaction centers (Fv/Fm), quantum efficiency of PSII (ΦPSII), non-photochemical quenching (NPQ), photochemical quenching (qP) and electron transport rate (ETR), which defines the overall performance of plants under different stresses [27]. Salt-resistant plants are known to possess resilient thylakoid reactions to overcome salinity effects such as photodamage [28] and protection of the reaction centers [29]. This may include protective mechanisms such as cyclic electron flow, photorespiration in C3 plants and regulation of NPQ [18][30]. CO2 fixation and thylakoid reactions of photosynthesis take place in thylakoids and the stroma of the chloroplast, providing the essential carbon skeleton for growth, energy for driving various metabolic reactions as well as the biosynthesis of different metabolites. Salt-induced toxicity negatively affects all these processes, resulting in poor plant growth and reduction in yield. Chloroplasts are also major reactive oxygen species (ROS) production sites at the reaction centers of PSII and PSI, where charge separation occurs, and the electron transport chain (ETC) from PSII to PSI are highly sensitive to salt-induced toxicity under which ROS production is further increased [31]. Higher concentrations of ROS cause oxidative damage to membranes, lipids, nucleic acids, proteins and some photosynthetic enzymes, resulting in reduced CO2 fixation, slower plant growth and consequently low crop yields. The ROS-scavenging system includes both enzymatic and non-enzymatic antioxidants that prevent oxidative damage. Therefore, manipulation of the components of this system holds great implications for improving the photosynthetic rates under salt stress in crop plants. This has been tested by overexpression of Cu/Zn superoxide dismutase (SOD) in the chloroplasts of tobacco [32][33] and Chinese cabbage [34]. Since chloroplasts are largely under the control of nuclear gene expression for growth and metabolic activities, chloroplasts have evolved a sophisticated signaling network to coordinate with the nucleus to control gene expression and maintain the balanced expression of genes in the two compartments. Chloroplasts also act as global sensors relaying changes in their own developmental status as well as in the environmental conditions, including light intensity and stresses to the nucleus. As a result, the nucleus adjusts the expression of its genes to ensure optimal plant performance under changing environmental conditions [35]. Until recently, this chloroplast–nucleus communication has been largely viewed as bilateral, ignoring the pivotal role of chloroplasts in adjusting gene expression and metabolic processes that affect photosynthesis and ultimately crop yields.
2. Effects of Salinity Stress on Chloroplast Structure and Function
Soil salinity is one of the major challenges to the sustainable development of agriculture in different parts of the world. Salinity has detrimental effects on plant growth by imposing several constraints. For instance, salt-induced toxicity impairs the normal functioning of the organelles, such as chloroplasts—the green plastids—which house several important biochemical reactions, including photosynthesis. Chloroplast dysfunction as a result of various environmental stresses, including salinity, has been reported to have detrimental effects on plants [36]. Chloroplasts, in addition to being a site of various metabolic reactions, also act as global sensors to sense and communicate the developmental, operational and environmental changes to the nucleus.
Understanding the effect of salinity on chloroplast function and the response of various metabolic reactions to salt stress is necessary for the development of salt-tolerant crops. Little attention has been paid to how salinity affects chloroplasts and the stromal metabolic reactions. Salinity-related changes in the size, number, lamellar organization, lipid and starch accumulation, and trafficking across the chloroplast membrane are dependent on the plant species and its level of salt tolerance. Chloroplast swelling or alteration in thylakoid membranes of glycophytes may be linked with the ionic component of salinity while some halophytes are affected by the osmotic effect of high salinity (Figure 1). Most halophytes either maintain chloroplast structure or enhance grana development under salinity stress. Swelling of thylakoids and disruption of chloroplast envelopes in mesophyll cells along with intact chloroplasts in bundle sheath cells is a general C4 response under salinity, irrespective of the subtype.
Halophytes and glycophytes have evolved different pathways to respond to salinity stress. For example, halophytes are much better adapted at maintaining a lower salt concentration in the cytoplasm compared to glycophytes. Likewise, chloroplasts in halophytes seem to have a better antioxidant system than those of glycophytes, and consequently more protected photosynthetic apparatuses under salt stress. Similarly, salinity-triggered starch deposition appears to be a damage symptom in glycophytes but a survival strategy in halophytes. The salinity-induced influx of Na+ and Cl− appears beneficial for halophytes but lethal for glycophytes (Figure 1). Accumulation of Na+ or Cl− disrupts ionic homeostasis, impairs protein synthesis and interferes with the enzymatic activities of the organelle. However, recent work suggests that the negative effects of these ions on plant health are not because of toxicity per se but are the result of interference with the absorption or metabolism of other essential ions [28]. This view stems from the evidence that K+ influx in chloroplasts is reduced with excessive Na+ or Cl− accumulation. K+ is an essential element for the plant cell and is not only required for chloroplast development but also for pH regulation, maintenance of the electron transport chain and thylakoid restacking [28][29]. Osmolyte synthesis suggests that organic solutes may help in fine adjustment along with ion transport (vacuolar compartmentation) and accumulation of cytosolic K+ in stressed environments rather than osmotic adjustment. However, osmolytes are certainly involved in the osmoprotection of membrane transport proteins and the scavenging of ROS. Despite ion regulation and osmotic adjustment, salinity induces many changes in chloroplast functions and signaling.
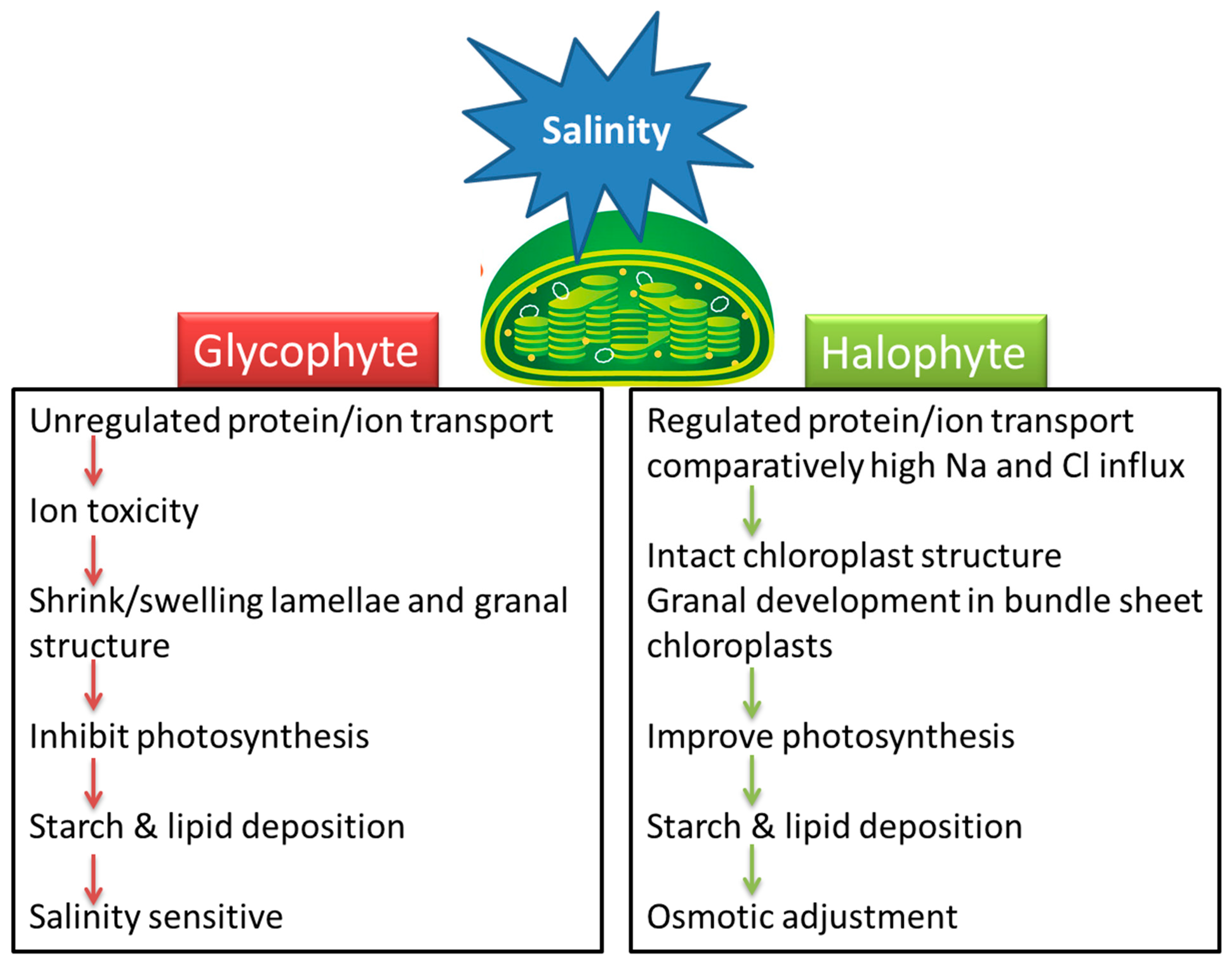 Figure 1. A model that summarizes the effects of salinity stress on chloroplasts in salt-sensitive (glycophyte) and salt-tolerant (halophyte) plants.
Figure 1. A model that summarizes the effects of salinity stress on chloroplasts in salt-sensitive (glycophyte) and salt-tolerant (halophyte) plants.Chloroplastic CO2 fixation is generally more sensitive to salinity than the thylakoid reactions. However, CO2 fixation in many halophytes is reportedly less prone to salinity compared to glycophytes. One major evolutionary adaptation that seems to operate in halophytes is the switching of CO2 concentration around Rubisco under stressful environmental conditions, including salinity. The reduced photosynthetic efficiency is considered a major salt-induced constraint inhibiting plant growth, and ultimately crop productivity. However, it is not yet clear whether the decrease in photosynthesis is the cause of growth reduction or the reduction in the growth rate causes a decrease in photosynthesis under salt stress. Nevertheless, a reduced rate of photosynthesis leads to higher production of ROS and also triggers the activity of ROS-scavenging enzymes. The higher activity of the ROS-detoxifying enzymes maintains a level of these species in a functionally useful range required for cell signaling. These enzymatic systems are naturally present in plants. Although differences in the activity of these enzymes have been reported in different genotypes, it is believed to be associated with responses such as stomatal closures, reduction in the CO2 fixation rates and an increase in photorespiration under stressful conditions [37][38]. Tight regulation of ROS alongside many chloroplastic metabolites also function as ‘putative’ signals for communication between chloroplasts and the nucleus (as well as other organelles) via so-called ‘retrograde signaling’. Despite information on crop and model plants, our knowledge about such signaling in halophytes is still far from full comprehension. Chloroplast functions, including photosynthesis, are integrated with other basic plant metabolic mechanisms of the plant in response to stresses, including salinity, and multiple factors work together to confer tolerance against salinity [39]. These factors include ion regulation that controls uptake and transport of salt and other ions to compartments within the plant cell, synthesis of compatible solutes, antioxidative enzymes and plant hormones and changes in photosynthesis and membranes in the cell [39]. Some of these occur within the chloroplast but are not limited to that location. These mechanisms are quite complicated, and many questions remain unanswered [39][40]. Some of these questions include how the plant senses salinity to initiate the signaling process, the precise details of how salinity stress leads to stomatal closure and growth reduction and the specific targets of ion toxicity in plant cells [40]. While advances are being made, a detailed understanding of the mechanisms behind salt tolerance is not yet clear. A comprehensive understanding of these mechanisms by employing multidisciplinary approaches is necessary for their effective incorporation into salt-sensitive crops for better crop yields under stressful environments.
This entry is adapted from the peer-reviewed paper 10.3390/cells10082023
References
- Hussain, T.M.; Chandrasekhar, T.; Hazara, J.; Sultan, Z.; Saleh, B.K.; Gopal, G.R. Recent advances in salt stress biology—A review. Biotech. Mol. Biol. Rev. 2008, 3, 8–13.
- Allakhverdiev, S.I.; Murata, N. Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth. Res. 2008, 98, 529–539.
- Akyol, T.Y.; Yilmaz, O.; Uzİlday, B.; Uzİlday, R.Ö.; Türkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13.
- Badawi, G.H.; Yamauchi, E.Y.; Shimada, R.; Sasaki, N.; Kawano, K.; Tanaka, K.; Tanaka, K. Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci. 2004, 166, 919–928.
- Jing, X.; Hou, P.; Lu, Y.; Deng, S.; Li, N.; Zhao, R.; Sun, J.; Wang, Y.; Han, Y.; Lang, T. Overexpression of copper/zinc superoxide dismutase from mangrove Kandelia candel in tobacco enhances salinity tolerance by the reduction of reactive oxygen species in chloroplast. Front. Plant Sci. 2015, 5, 23.
- Tseng, M.J.; Liu, C.-W.; Yiu, J.-C. Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol. Biochem. 2007, 45, 822–833.
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681.
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant 2013, 35, 2867–2878.
- Abdelhamid, M.T.; Sekara, A.; Pessarakli, M.; Alarcon, J.J.; Brestic, M.; El-Ramady, H.; Gad, N.; Mohamed, H.I.; Fares, W.M.; Heba, S.S.; et al. New approaches for improving salt stress tolerance in rice. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Roychoudhury, A., Ed.; Springer: Singapore, 2020.
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664.
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 2019, 39, 999–1014.
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J. Plant Growth Regul. 2020, 1–16.
- Ibrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Ziveak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M.; et al. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotechnol. 2021, 329, 180–191.
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963.
- Asrar, H.; Hussain, T.; Hadi, S.M.S.; Gul, B.; Nielsen, B.L.; Khan, M.A. Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ. Exp. Bot. 2017, 135, 86–95.
- Bellasio, C.; Quirk, J.; Beerling, D.J. Stomatal and non-stomatal limitations in savanna trees and C4 grasses grown at low, ambient and high atmospheric CO2. Plant Sci. 2018, 274, 181–192.
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84.
- Galmés, J.; Molins, A.; Flexas, J.; Conesa, M.À. Coordination between leaf CO2 diffusion and Rubisco properties allows maximizing photosynthetic efficiency in Limonium species. Plant Cell Environ. 2017, 40, 2081–2094.
- Hussain, T.; Huchzermeyer, B.; Koyro, H.-W.; Khan, M.A. Linkage between leaf development and photosynthetic response at hyperosmotic salinity in the C-4 grass Panicum antidotale. Flora 2019, 256, 52–60.
- Nunes-Nesi, A.; Nascimento, V.d.L.; de Oliveira Silva, F.M.; Zsögön, A.; Araújo, W.L.; Sulpice, R. Natural genetic variation for morphological and molecular determinants of plant growth and yield. J. Exp. Bot. 2016, 67, 2989–3001.
- Rasouli, F.; Kiani-Pouya, A.; Tahir, A.; Shabala, L.; Chen, Z.; Shabala, S. A comparative analysis of stomatal traits and photosynthetic responses in closely related halophytic and glycophytic species under saline conditions. Environ. Exp. Bot. 2021, 181, 104300.
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.M.; Skalicky, M.; Ibrahimova, U.F.; Hussain, S.; Mbarki, S.; et al. JIP-test as a tool to identify salinity toelrance in sweet sorghum genotypes. Phostosynthetica 2020, 58, 518–520.
- Zuo, Z.; Ye, F.; Wang, Z.; Li, S.; Li, H.; Guo, J.; Mao, H.; Zhu, X.; Li, X. Salt acclimation induced salt tolerance in wild-type and chlrophyll b-deficient mutant wheat. Plant Soil Environ. 2021, 67, 26–32.
- Tomeo, N.J.; Rosenthal, D.M. Variable mesophyll conductance among soybean cultivars sets a tradeoff between photosynthesis and water-use-efficiency. Plant Physiol. 2017, 174, 241–257.
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096.
- Rodrigues, N.F.; da Fonseca, G.C.; Kulcheski, F.R.; Margis, R. Salt stress affects mRNA editing in soybean chloroplasts. Genet. Mol. Biol. 2017, 40, 200–208.
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017.
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143.
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825.
- Gulzar, S.; Hussain, T.; Gul, B.; Hameed, A. Photosynthetic Adaptations and Oxidative Stress Tolerance in Halophytes from Warm Subtropical Region; Springer: Cham, Switzerland, 2020.
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668.
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190.
- Koyro, H.-W.; Hussain, T.; Huchzermeyer, B.; Khan, M.A. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environ. Exp. Bot. 2013, 91, 22–29.
- Loreto, F.; Centritto, M.; Chartzoulakis, K. Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell Environ. 2003, 26, 595–601.
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2013, 65, 1241–1257.
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396.
- Turkan, I.; Uzilday, B.; Dietz, K.J.; Bräutigam, A.; Ozgur, R. Reactive oxygen species and redox regulation in mesophyll and bundle sheath cells of C4 plants. J. Exp. Bot. 2018, 69, 3321–3331.
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotox. Environ. Saf. 2005, 60, 324–349.
- Isayenkov, S.V.; Maathuis, J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80.
This entry is offline, you can click here to edit this entry!
