Gastrointestinal (GI) cancer is a prevalent global health disease with a massive burden on health care providers. Internal and external factors such as obesity, smoking, diet (red meat), low socioeconomic status and infection with Helicobacter pylori are the critical risk factors of GI cancers. Flavonoids are natural phenolic compounds found abundantly in fruits and vegetables. Upon ingestion, 90% of flavonoids consumed require further enzymatic metabolism by the gut microbiome to enhance their bioavailability and absorption. Several epidemiological studies reported that consumption of flavonoids and their enzymatic conversion by gut microbes is strongly associated with the reduced risk of GI cancer development. This review summarizes the current knowledge on the enzymatic conversion of flavonoids by the human gut microbiome. It also addresses the underlying anti-GI cancer effects on metabolic pathways such as apoptosis and cellular proliferation. Overall, metabolites produced from flavonoid’s enzymatic conversion illustrate anti-GI cancer effects, but the mechanisms of action need further clarification.
- gastrointestinal cancer
- flavonoids
- gut enzymes
- microbiome
- anticancer
1. Introduction
1.1. Gastrointestinal Cancer
1.2. Flavonoids and Cancer
1.3. Flavonoids Metabolism in the Gut
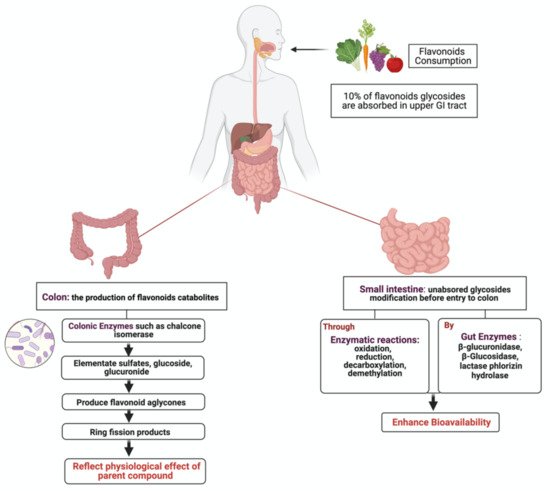
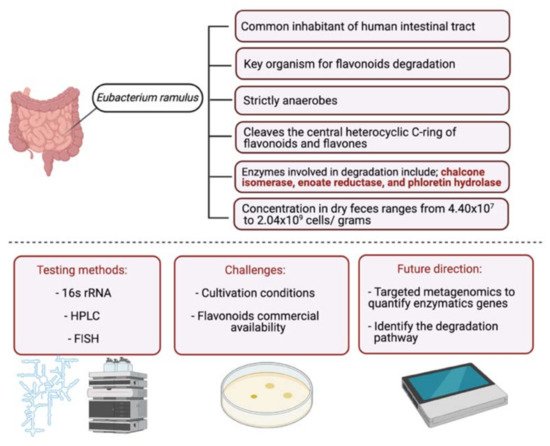
3. Metabolism of Flavonoids by Gut Microbiota
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced by Gut Microbiota | Bacteria Involved in Metabolism | Enzymes Involved in Metabolism | Site of Metabolism | Conversion Mechanism | Effect of Microbiota on Flavonoids | Model Used | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||||||
| Flavonol | 1. Rutin | 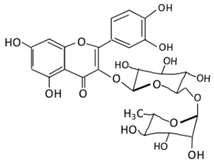 |
Lemons, berried, limes and oranges |
Quercetin -3- O- glucoside Quercetin |
Lachnoclostridium Eisenbergiella Escherichia Parabacteroides Erysipelatoclostridium |
α-rhamnosidases β-glucosidases |
Colon | Hydrolysis of rutin to remove sugar moiety | Permit the absorption of the aglycone Enhance bioavailability |
10 fecal samples from healthy individual following omnivore diet. |
[29,30,31,32,33] | ||
| 2. Fisetin | 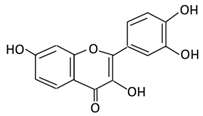 |
Persimmon and onions | No available data | [34,35] | |||||||||
| 3.Kaempferol | 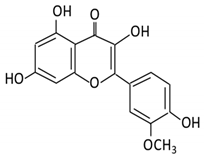 |
Tea, berries and cruciferous vegetables |
Kaempferol -3-O- glucoside p-coumaric acid kaempferol 3-(4 hydroxyphenyl) propionic acid 3-phenylpropionic acid |
Lactobacillus paracasei A221 |
β-glucosidases | Intestinal tract | (A)Degradation through multiple chemical reactions: - Deglycosylation - Reduction - Dehydroxylation (B) Hydrolysis |
Permit the absorption of the aglycone Enhance bioavailability |
Mice | 3 fecal samples from healthy individual Caco-2 intestinal barrier model |
[36,37,38,39] | ||
| 4. Quercetin | 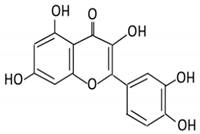 |
Black currants, cherries, apples and chokeberries |
4-hydroxybenzoic acid 3,4-dihydroxyphenylacetic acid 3,4-dihydroxybenzoic 3-(3-hydroxyphenyl) propionic acid |
Bacteroides fragilis Clostridium perfringens Eubacterium ramulus Streptococcus S-2 Lactobacillus L-2 Bifidobacterium B-9 Bacteroides JY-6 |
Lactate phlorizin hydrolase | Small intestine |
Deglycosylation | Enhance bioavailability | Mice | 87 fecal samples from healthy elderly individual |
[40,41,42,43,44,45] | ||
| 5. Isorhamnetin | 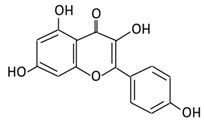 |
Ginkgo biloba and Hippophae rhamnoides |
3-O-neohesperidoside Isorhamnetin -3- glucoside Aglycone isorhamnetin |
Escherichia Enterococcus Bacillus. |
Not identified | Small intestine |
Deglycosylation | Permit the absorption of the aglycone | Rats | 1 fecal sample from healthy female |
[46,47] | ||
| 6. Morin | 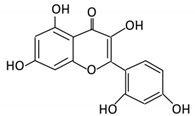 |
Psidium guajava, Prunus dulcis (Almond), chlorophora tinctoria and fruits |
Morin glucuronides Morin sulfates |
No available data | [12] | ||||||||
| Flavanones | 7. Hesperidin | 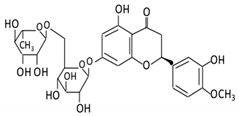 |
Orange citrus aurantium | Hesperetin | Not identified | Rutinose | Large intestine |
Cleaves the attached rutinose moiety |
Permit the absorption of the aglycone Enhance bioavailability |
fecal/ blood samples from 18 Lewis male rats |
[48,49,50,51] | ||
| 8. Naringenin | 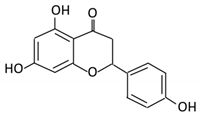 |
C.aurantium (chinese herbs) and grapefruit | Aglycone naringenin | Ruminococcus gauvreauii Bifidobacterium catenulatum Enterococcus caccae Eubacterium ramulus |
Chalcone isomerase | Large intestine |
Remove sugar group | Permit the absorption of the aglycone Enhance bioavailability |
fecal samples from healthy individuals |
[52,53,54] | |||
| 9. Eriodictyol | 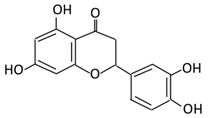 |
Lemon (Eriocitrin), Torr, Eridictyon californicum, | Eriodictyol 3,4-dihydroxyhydrocinnamic acid Phloroglucinol |
Parabacteroides distasonis Bacteroides uniformis JCM 5828 |
Chalcone isomerase | Colon | O-Deglycosylation | Permit the absorption of the aglycone Enhance bioavailability |
fecal samples from healthy individuals | [55,56,57] | |||
| Isoflavones | 10. Genistein | 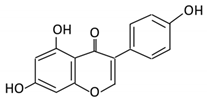 |
Fava and soy beans | Dihydrogenistein 6-hydroxy-O-desmethylangolensin 2-(4-hydroxyphenyl) propionic acid |
Lactobacillus Eubacterium ramulus |
Lactate phlorizin hydrolase | Small intestine |
Fermentation by anerobic bacteria | Permit the absorption of the aglycone Enhance bioavailability |
Samples from C57BL/6 female mouse |
[58,59,60,61] | ||
| 11. Daidzein | 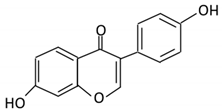 |
Soybeans, nuts and soymilk | Dihydrodaidzein O-desmethylangolensin S- equol |
Clostridium-like strain | β- glucosidase Lactate phlorizin hydrolase |
Colon | Reduction | Permit the absorption of the aglycone Enhance bioavailability |
Mice | [62,63,64] | |||
| Anthocyanins | 12. Cyanidin | 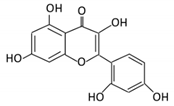 |
Bilberry, blueberry, grapes, blackberries, hawthorn | Cyanidin-3- glucoside | Clostridium saccharogumia Eubacterium ramulus |
Combined activity of bacteria and host enzymes | Small intestine |
Degrade polyphenolic glycosides |
Permit the absorption of the aglycone Enhance bioavailability |
Dawley rats | Fecal samples from healthy individuals | [65,66,67] | |
| 13. Delphinidin | 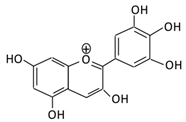 |
Dark grapes, eggplant, berries, red cabbage, | Gallic acids | Lactobacillus | β-d-glucuronidase β-d-glucosidase α-rhamnosidase α-galactosidase |
Colon | Cleavage of glycosidic bonds | Permit the absorption of the aglycone Enhance bioavailability |
Mice | [68,69,70,71,72] | |||
| 14. Pelargonidin | 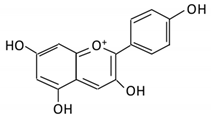 |
Bilberry and ficus bengalensis Linn | 4-hydroxybenzoic | Lactobacillus | β-d-glucosidase β-d-glucuronidase α-galactosidase α-rhamnosidase |
Colon | Cleavage of glycosidic bonds | Permit the absorption of the aglycone Enhance bioavailability |
Mice | [73,74] | |||
| Flavones | 15. Baicalein | 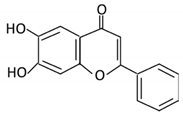 |
Scutellaria lateriflora L | Baicalein | E. coli | β-glucuronidase | Intestine | Hydrolysis to remove moiety |
Permit the absorption of the aglycone Enhance bioavailability |
Mice | HCT-166 cells SW-480 cells |
[75,76,77,78] | |
| 16. Luteolin | 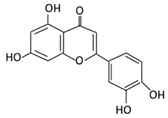 |
broccoli, celery and parsley, | No available data | [12] | |||||||||
| 17. Diosmin | 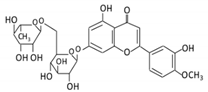 |
Citrus fruits | Diosmetin | Not identified | α- glucosidase β- glucosidase |
Small intestine |
Hydrolysis | Enhance bioavailability | Blood samples from healthy participants |
[79,80,81,82] | |||
| 18. Apigenin | 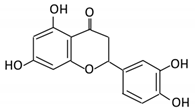 |
Tea, chamomile, parsley and oranges |
3-(4-hydroxyphenyl) propionic acid Apigenin |
Bacteroides distasonis Eubacterium ramulus Clostridium orbiscindens |
β- glucosidase lactase-phlorizin hydrolase |
Small intestine |
Glucuronidation Hydrolysis |
Enhance bioavailability |
Rats | Fecal and urine samples | [83,84,85,86] | ||
| 19. Tangeretin |  |
Poncirus trifoliate L, citrus fruit | Tangeretin-O-glucuronides | Lactobacillus Bifidobacterium |
Possibly by: A) rhamno glucosides B) C- glycosyl |
Small intestine |
Demethylation | Permit the absorption of the aglycone |
Rats | [87,88] | |||
| 20. Wogonin |  |
Scutellaria baicalensis Georgi | Wogonin | Not identified | β-glucuronidase | Intestine | Hydrolysis | Enhance absorption and bioavailability | Sprague-Dawley rats | [89,90,91,92] | |||
| 21. Chrysin |  |
Honey | Chrysin glucuronides | Flavonifractor plautii ATCC 49531 | Flavone reductase |
Intestine | Reduction | catalyzes the hydrogenation of the C2–C3 double bond | ATCC 49,531 strain | [93,94,95] | |||
2.1. Flavonol
2.1.1. Rutin
2.1.2. Fisetin
2.1.3. Kaempferol
2.1.4. Quercetin
2.1.5. Isorhamnetin
2.2. Flavanones
2.2.1. Hesperidin
2.2.2. Naringenin
2.2.3. Eriodictyol
2.3. Isoflavones
2.3.1. Genistein
2.3.2. Daidzein
2.4. Anthocyanins
2.4.1. Cyanidin
2.4.2. Delphinidin
2.4.3. Pelargonidin
2.5. Flavones
2.5.1. Baicalein
2.5.2. Diosmin
2.5.3. Apigenin
2.5.4. Tangeretin
2.5.5. Wogonin
2.5.6. Chrysin
4.1. Flavonoids Effects on Impaired General Pathways
4.1.1. Apoptosis
A process described as programmed cell death is characterized by the changes in the biochemical mechanisms and the morphological characteristics of the cell [142]. It is considered a critical component of various biological processes such as embryonic development, chemical-induced cell death and normal cell turnover [143]. A wide variety of pathological and physiological stimuli and conditions could trigger apoptosis [144]. Unregulated apoptosis can result in neurodegenerative, autoimmune diseases and cancer [145]. In cancer, apoptosis is considered the hallmark for cell survival, invasiveness and cellular proliferation. There are multiple ways in which cancer cells can evade intrinsic and extrinsic apoptotic pathways, such as inhibition of caspase function and upregulation of anti-apoptotic BCL-2 proteins [146].
Consumption of flavonoids is reported to induce apoptosis in GI cancer, acting as a potential therapeutic agent [147]. Flavonoids could induce apoptosis by acting on the intrinsic apoptotic pathway, as in the case of fisetin. Gastric cancer cells treated with (25-100 μM) of fisetin induced apoptosis by dissipating mitochondrial potential and upregulating pro-apoptotic molecules such as Bcl-2 and tumor suppressors such as P53 [148]. Additionally, other flavonoids such as cyanidin upregulate the expression of caspase 3, therefore activating the extrinsic apoptotic pathway in human gastric adenocarcinoma cells [127]. While some flavonoids target one specific pathway, other targets both intrinsic and extrinsic pathways such as apigenin in colorectal cancer cells or, as in the case of hesperidin, flavonoids can target multiple components of the same pathway [119,135]. Figure 3 summarizes reported flavonoids that induce apoptosis of GI cancer cells and their target’s key components.
4.1.2. Cellular Proliferation
Cellular proliferation is a fundamental process for homeostasis and cellular development that is tightly regulated to ensure accurate genome duplication[149,150]. Phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is one of the most critical intracellular pathways to regulate cellular survival, growth, motility and metabolism [151]. Under baseline conditions, PI3K is activated by an external stimulus such as cytokines, hormones and growth factors. Upon activation, phosphorylation yields a second messenger (PIP3) that binds and recruits lipid-binding domains that target cell membrane. Signaling proteins such as AKT kinase binds to PI3K to activate cellular growth [152]. Phosphatase and tensin homolog (PTEN) regulate the pathway by the dephosphorylation of PI3K, thus preventing downstream activation [153]. In cancer, PI3K pathway can be downregulated by the inactivation of PTEN (tumor suppressor), mutation of PI3K and activation of tyrosine kinase [154].
Flavonoids such as hesperidin can inhibit the proliferation of cancer cells [155]. In an in vivo and in vitro study, the results provided strong evidence that hesperidin enhances antitumor effects on gastric cancer by regulating PI3K/AKT signaling pathway through the upregulation of PTEN expression. In addition, a combinatory therapy of cisplatin with hesperidin could enhance the clinical outcome [120]. Additionally, luteolin can inhibit cellular proliferation by regulating PI3K, AKT and mTOR signaling pathways, which play a key role in the progression and development of gastric cancer [132]. Figure 4 highlights the reported activities of flavonoids affecting GI cancer cells proliferation.
5. Discussion
5.1. Clinical Implementation of Flavonoids
As illustrated in Section 4, flavonoids implementation in GI cancer treatments has tumor-suppressive activities in theory. To effectively use flavonoids in cancer therapy, clinical trials are required to assess the impact of flavonoids subclass on GI cancer and gut enzymes. In 2003, flavopiridol, a synthetic flavone reported to inhibit cell cycle progression, was evaluated in 20 patients with advanced colorectal cancer. The patients received flavopiridol at a dose of 50 mg/m2/day every 14 days via continuous infusion for eight weeks. The phase II clinical trial results reported minimal hematological activities and moderate diarrhea and fatigue in the 20 patients. Even though the pre-clinical data showed promising antitumor activities, no impartial response was observed as only 28% of the patients experienced stabilization of the disease [174]. Limited studies are reported in the literature, and more are required to evaluate the appropriate dose-administered, the appropriate stage of the disease that tolerates flavonoids administration, and the impact of combination therapy (flavonoids and chemotherapy or a mixture of flavonoids) have synergistic effects on gut enzymes and GI cancer.
5.2. Impact of Current Cancer Treatment on Gut Enzymes
The field of modern oncology has produced significant advances in cancer treatment, improving and prolonging patient’s lives [175]. Due to observed long-term side effects on cancer survivors, cancer microbiome research that addresses the crucial role of the gut microbiome in improving the efficacy of cancer therapy is rapidly emerging [176]. Chemotherapy can have devastating effects on microbial diversity leading to gastrointestinal toxicities, acute dysbiosis and delaying the response to treatment. Modulation of the gut microbiome was suggested as a practical therapeutic approach to improving cancer treatment’s toxic side effects [177]. Lactobacillus and Bifidobacterium along with one digestive enzyme were evaluated for their efficacy in protecting the GI tract after chemotherapy treatment. The results showed an improvement in the colon’s fermentation process and the recovery of microbial population in which the ratio of Bacteroidetes to Firmicutes was restored, inducing microbial metabolites production and enhancing anti-inflammatory response [178].
Moreover, a combination of rutin and chemotherapeutic agent Oxaliplatin promoted apoptosis of gastric cancer cells SGC-7901 demonstrated through decreased BCL-2/Bax ratio while the apoptotic mechanisms of rutin were related to caspase-mediated signaling [179]. In addition, luteolin combined with Oxaliplatin, suppressed proliferation and induced apoptosis in gastric cancer SGC-7901 cells through cleaved caspase-3, upregulated Bax and downregulated Bcl-2 [133]. More efforts are required to address the impact of cancer treatment on gut enzymatic activities (enhancement/depletion) and natural product metabolism.
5.6. Possible Synergistic Effects of Flavonoids?
While the type and concentration of flavonoids consumed are critical to observe the possible biological activities, selecting the ones that trigger multiple metabolic pathways may be improve the pathogenesis of GI cancer. For instance, genistein, an isoflavone, triggers four different pathways in cancer such as the extrinsic apoptotic pathway by activating caspase 3 activates, cellular invasion by enhancing the expression of E- cadherin, cellular proliferation by an unknown mechanism, and cellular inflammation by reducing nuclear translocation of NF-κB. On the other hand, baicalein, delphinidin and daidzein act on more specific pathways (Figures 7–9). By combining these four flavonoids together, could their anti-cancer effects improve as they targeted multiple pathways? Moreover, could they complement each other pathway-wise? Currently, these are only suggestions that need further research to support and avoid possible side effects. Also, more research is needed to investigate the mechanism of hesperidin, a flavanone, which triggers both intrinsic and extrinsic apoptotic pathways.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13163934
