Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
|
Cell & Tissue Engineering
Four sensory systems (vestibular, lateral line, electroreception, auditory) are unique and project exclusively to the brainstem of vertebrates. All sensory neurons depend on a common set of genes (Eya1, Sox2, Neurog1, Neurod1) that project to a dorsal nucleus and an intermediate nucleus, which differentiate into the vestibular ear, lateral line and electroreception in vertebrates.
- neurons
- brainstem nuclei
- hair cells
- bHLH genes
- Sox2
- Eya1
- Lmx1a/b
1. Introduction
Sensory maps depend on the specific sensory modality and the relevant information to be extracted by them. Beyond primary sensory maps, central map formation underlies the integration of various sensory modalities, namely the ear, lateral line and electroreception. The four primary sensory maps of vertebrates have unique features and seemingly use distinct molecular cues, cell cycle exit and activity combinations during development, regeneration and plasticity. The evolution of chordates is comparable with the organization of the dorsal spinal cord and brainstem, which is associated with neurons and hair cells in 71,000 vertebrates. On the other hand, we have limited support for the two chordates associated with the neural crest and placodes, hair cells and central brainstem in 31 species of lancelets and 3100 species of ascidians. Fossils appeared approximately 540 million years ago (Mya), and all major bilaterian phyla presented by 500 Mya [1].
The brainstem of vertebrates is organized into rhombomeres (r0-11) that superficially resemble other chordates, lancelet and ascidians [2,3,4]. A dorsal part of the brainstem expresses a continuation to the spinal cord in vertebrates [5] which is absent in a true brainstem in other chordates. Partial similarity is found in ‘dorsal root ganglia’ in ascidians that resembles the spinal cord in vertebrates, which is absent in lancelets [2,6,7]. Adding these differences in chordates, gene duplication [8], followed by diversification [9,10], is the basis for the unique brainstem, neurons and hair cells that developed in vertebrates [11]. The unique formation of mechano- and electroreception evolved in four distinct sensory inputs that are partially similar with the lateral line of ascidians [6,12,13,14]. The progression must start with the sensory neurons that connect all neurons with the brainstem and reach out the peripheral sensory hair cells.
Neurons depend upon Eya1 [15], Sox2 [16], Neurog1 [17] and Neurod1 [18]. In contrast to Neurog1 null mice, which showed a complete loss of neurons [19], Neurod1 null mice showed residual neurons extending centrally to smaller vestibular and cochlear nuclei [20,21] that reached the ear [22,23]. It is worth noting that the lateral line and electroreception are separate for the vertebrate ear that is lost in most tetrapods to generate novel cochlear neurons, the spiral ganglion neurons (Figure 1).
The brainstem is a continuation of the spinal cord (SC; [11,24,25]) that develops into rhombomeres and differentiates into nuclei, namely the vestibular, lateral line and electroreception nuclei in basal vertebrates (Figure 1). Loss of the lateral line and electroreception leads to the development of cochlear nuclei in tetrapods [26,27]. All dorsal expression of the brainstem depends on Lmx1a/b [28] and Gdf7 [29], which drive the choroid plexus (Figure 1). Combined, Lmx1a/b and Gdf7 regulate the formation of Wnt1/3a, BMP4/7 and Atoh1. This formation is likely reduced or absent in Neurog1/2, Ascl1, Ptf1a and Olig3, among others (Figure 1).
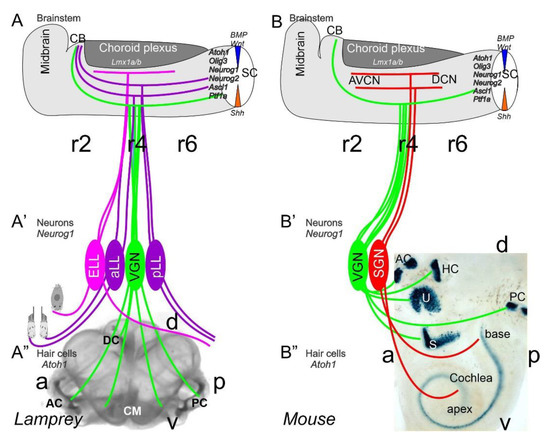

Figure 1. Inner ear, lateral line and electroreception revealed. Neurons (Neurog1; A’) form vestibular ganglia (VGN) to reach out 4 hair cell organs in lampreys (A”). A separate lateral line (LL) and electroreceptor neurons (ELL) that innervate hair cells project more dorsal in lampreys. Central projection depends on Atoh1 to receive LL and ELL fibers, whereas several bHLH genes (Neurog1/2, Olig3, Ascla1, Ptf1a) receive all VGN (A). In the absence of ELL and LL development in amniotes, mammals develop separate spiral ganglion neurons (SGN; B’) that extend from the cochlea (B”) and end in a topological central projection that depends on Atoh1 (B). The formation of VGNs (Neurog1; B’) reach the 5 hair cells (B”) to extend the distribution of bHLH genes. Note that certain areas are lost or gained which enter central projections near r4. Images are shown by miR-183 ISH (A”) and Atoh1-LacZ (B”). AC, anterior crista; AVCN, anteroventral cochlear neurons; CB, cerebellum; aLL, pLL, anterior/posterior lateral line neurons; CM, common macula; DC, dorsal crista; DCN, dorsal cochlear neurons; HC, horizontal crista; PC, posterior crista; r2/4/6, rhombomeres; S, saccule; SC, spinal cord; U, utricle. Modified after [11,30,31].
Mechanosensory and electrosensory hair cells (Figure 1) depend on Eya1, Sox2 and Atoh1 to initiate the cell cycle and to differentiate into vestibular, cochlear, lateral line and electrosensory hair cells [22,32,33]. Planar cell polarity (PCP) depends on the formation of shifting the central projection of the kinocilium into a lateral position. PCP extends the length of the stereocilia to develop the staircase of tip links of the vestibular, cochlear and lateral line hair cells [34,35,36]. The next step involves the development of the tip links to allow the connections between CDH23 and PCDH15 to open up the channel to form a mechanosensory hair cell [37,38], with opposing polarity in most of the ear and lateral line [34,39,40,41]. TMC1/2 provides a major function that seems to interact with additional channel proteins (TMHS, TMIE), forming a complex interaction [37,42,43,44]. In contrast, while the electroreception forms next to lateral line hair cells [22,23,45], these hair cells lack any polarity organization, and certain ampullary hair cells are dependent on Cav1.3 [46].
2. Neurons Depend upon Eya1, Sox2, Neurog1 and Neurod1
The ear, lateral line and electroreception neurons depend on genes that, collectively, define their development. Upstream of bHLH genes, which initiate the proliferation of neurons, is the expression of Eya1, which interacts with Brg1 to initiate pro-neurosensory development [15,48,49]. In the absence of Eya1, there is no neuronal development that allows ear formation, and neither neurons nor hair cells differentiate [15]. Evolving neurons start in the lancelet, which lack dorsal root ganglia. The dorsal root ganglia show partial expression of Neurog inside the spinal cord (Figure 2), which lacks an Atoh gene [50,51]. In contrast, at least a smaller set of bHLH genes are partially characterized in the developing ascidian, Ciona [52], which have at least six bHLH genes driving neuron development: Ptf1a, Tcf3, Atoh, Ascl and Neurog [7,12]. A detailed serial section analysis shows the innervation of sensory cells (Atoh) from fibers of the neurons (bipolar tail neurons; Figure 2) that can trace to reach the anterior motor ganglion [13]. Neither the full expression of Eya nor Sox2 outside the neural plate are unclear in the lancelet and tunicates [2,52].
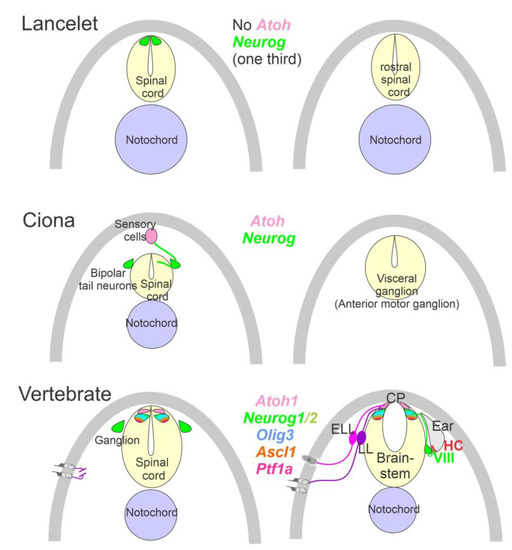
Figure 2. Neurons require Neurog expression. Lancelets have a limited description of bHLH genes that are characterized in the more caudal spinal cord, which is positive for Neurog. Note that the lancelet has no Atoh bHLH gene. Ciona has at least 6 bHLH genes expressed in sensory cells that are innervated by bipolar tail neurons which extend to reach the visceral ganglion for interactions. Atoh and Neurog genes are described in Ciona associated with the spinal cord. Vertebrates have dorsal root ganglia that depend on Neurog1/2, which is also expressed in Atoh1 and Neurog1 of the spinal cord. The brainstem is innervated by electroreceptor (ELL) and lateral line fibers (LL) that extend to innervate migration populations of LL and some ELL). The ear is unique in vertebrates, which give rise to the VIII ganglia that innervate more ventral nuclei compared to LL and ELL projections to reach Atoh1. CP, choroid plexus. Modified after [2,7,12,23,24].
A crucial next step is the initiation of Sox2, which is needed to upregulate Neurog1 [53,54,55]. In fact, Sox2 delays certain neuron development in bony fish [56], and in the presence of Sox2 is unclear the sequence of gene regulation in the lamprey and hagfish [57]. There is a distinct effect of the loss of early genes in the vestibular ganglion, which initially differentiates in the absence of Sox2 and Neurog1 (Figure 1 and Figure 2) and does not develop in the auditory neurons [16]. A loss of all auditory neurons, and partial loss of vestibular neurons, are known for Pax2 [58], Gata3 [59], Lmx1a/b [28], Fgfr2 [60], Shh [61] and Dicer [62]. Partial loss of some vestibular neurons are known for Fgf10 [63] and Foxg1 [64,65], indicating a limited loss of sensory hair cells and/or neurons. Unfortunately, the details of the lateral line and electroreception (Figure 1, Figure 2 and Figure 3) are not as fully genetically characterized [22,23,27,33]. The lateral line and electroreception likely depend on neuronal development (Figure 1 and Figure 2), including the development of spinal ganglia neurons [66] and trigeminal neurons [67,68,69]. A separate placode is derived from neurons that develop from Neurog2 in mammals [68,70]. In birds, this placode is driven by Neurog1 [71,72]. Furthermore, separate amniotic paratympanic placodal neurons innervate separate hair cells that partially integrate into the central vestibular projection [72].
In addition to directly initiating the formation of neurons by Eya1, Sox2, Pax2 and Neurog1/2, another set of genes are regulated to differentiate into Neurod1 [18,20,21,71,73], followed by Isl1, Foxg1, Pou4f1 and Phox2b [71,74,75,76], which interact with Shh, BMPs and Wnts to define neurons [77,78]. Regional regulation of the distinct vestibular, lateral line, electroreception and auditory neurons are sorted out by downstream genes regulating the distinct innervation. For example, the expression of Calbindin, Calretinin, Pou4f1 and Peripherin is required to sort out the innervation from the inner and outer hair cells [79,80,81,82]. In Sox10 null mice, an interaction showed disorganized cochlear neurons, whereas the development of vestibular neurons was near normal [83]. This interaction is consistent with the loss of Erb2 of nearly all cochlear neurons, as well as reduced vestibular neurons [84]. The concept of having multiple sources of neurons from the placode and neural crest is likely due to a misinterpretation [3,83,85,86,87].
Downstream of gene development, the expression of TrkB (Ntrk2) and TrkC (Ntrk3) has a reduction and loss in vestibular and cochlear neurons. Vestibular neurons are mostly dependent on TrkB [88,89] whereas the cochlear neurons are mostly dependent on TrkC [90,91]. Loss of both neurotrophin receptors causes the early loss of all neurons [92,93,94]. Limited expression is characterized in some ascidians which are unknown in the lancelet [1]. The comparable expression of the lateral line and electroreception are unclear due to the multiplication of neurotrophins in bony fish [95,96].
The proliferation of neurons and hair cells depend on MycN [97,98], which drives the division of the G1, S and G2 phases with a set of genes that interactions with cell cycle regulation [53,99,100,101]. Detailed characterization and proliferation have been described in the ear and brainstem, clarifying cell cycle progression in mice and rats [102,103,104]. Sox2 and Neurog1 are in negative feedback, which allows proliferation and initiates differentiation. This differentiation interacts with retinoblastoma (Rb), Hes/Hey and IDs to regulate the cyclin-dependent kinases (CDKs), cross-react with e-proteins and define whether a cell cycle is progressing [98,100,105,106]. In the end, continuation depends on either knocking out Rb to continue proliferation or upregulating of Sox2 to jumpstart proliferation [107,108].
In various vertebra, the central projection has been described to show the projection of the vestibular, lateral line, electroreception, and cochlea [3,67,87,109,110,111]. Three sets of central projections are known in vertebrates that develop a loss of the lateral line, electroreception and added cochlear nuclei [23,26,112]. For electroreception, these central projections always have a single set of an anterior ganglia (Figure 1 and Figure 3) that adds variably the electroreception in bony fish [27,113]. Lateral line neurons (Figure 1, Figure 2 and Figure 3) can be split into an anterior and posterior branch that diversify the neuromasts to innervate all lateral line hair cells (Figure 3; [114,115,116]). Vestibular neurons have two neuron populations in hagfish [57], while lampreys and jawed vertebrates have a single vestibular ganglion [111,117,118]. At least 4-5 distinct innervations are described in lampreys [119,120], whereas most gnathostomes have at least five and up to nine branches of vestibular and auditory connections (Figure 1 and Figure 3): three canal cristae, utricle, saccule, lagena, basilar papilla, amphibian papilla and neglecta [121,122]. Branches of discrete neurons are known for an anterior and a posterior (superior) nucleus that innervates two canal cristae (anterior and horizontal cristae), the utricle and part of the saccule (Figure 3). The remaining part of the utricle provides a posterior canal and the branch of the saccule (Figure 1 and Figure 3) in mammals [123]. The development of central projections follows a simple layout. First, the trigeminal and epibranchial neurons develop. Then, central projection follows. Subsequently, vestibular, lateral line and electroception develop, if present (Figure 3; [3,124]). Different developmental patterns exist in neuronal proliferation: nearly all neurons continue proliferation for a long time or lifetime, whereas mammals have an early production of neurons that ends proliferation very early [67,125,126]. The topology of peripheral neurons of the vestibular, lateral line and electroreceptors is unclear, suggesting an overlap with an incomplete segregation of neurons that is well known for the vestibular neurons (Figure 3 [123]).
A long-term proliferation of the vestibular, lateral line and electroreception is followed by a delayed formation of cochlear neurons, the spiral ganglia neurons (SGN), which follow vestibular neurons in mammals (vestibular neurons: E9-11; SGN: E10-12 [125,127]). A unique topological development is known among mammals [128], first showing the basal turn neurons (Figure 3), which reach the anteroventral, posteroventral, and dorsal cochlear nuclei (AVCN, PVCN, DCN). The development of these neurons is followed, with delay, by the apical neurons [67,87,110,129]. Interestingly, there are central projections that can form independently to reach the formation of cochlear nuclei [130]. In the absence of target hair cell development [92,131], cochlear neurons develop and largely proliferate prior to cochlear nuclei and cochlear hair cells (Figure 3). Central cochlea require the expression of Neurod1, Wnts, Fzd, Npr2 and Ephrins for targeted central projections [21,129,132,133].
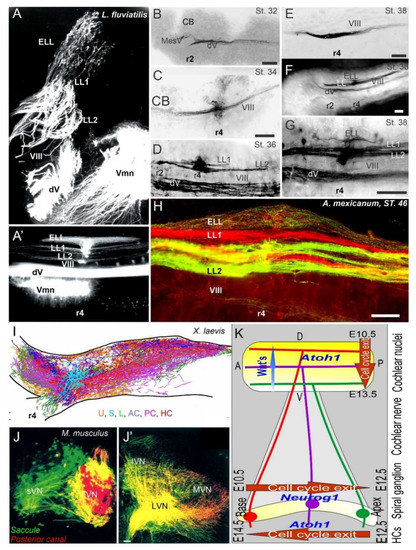

Figure 3. Central projections form afferents to distinct innervation. The lateral line of 2 or more branches form, whereas electroreception receives the short dorsal projection in lampreys (A,A’) and salamanders (B–H). Vestibular projection forms after the trigeminal central projection, followed by the lateral line and electroreception (B–H). Central projection in a frog (I) and mammal (J,J’) show the incomplete distribution of distinct neurons (J) that overlap and incompletely segregate the vestibular projection (I,J’). Spiral ganglia (K) proliferate neurons in a base to apex progression (E10.5-12.5) that reach the central projection to form a topology from dorsal to ventral cochlear nuclei (E10.5-13.5), depending on Wnt expression. Later, hair cells proliferate from apex to base (E12.5-14.5) that reach the afferents. AC, anterior crista; dV, trigeminal afferents; ELL, electroreception; HC, horizontal crista; LL1/2; lateral line; L, lagena; LVN, lateral vestibular nuclei; IVN, inferior vestibular nuclei; iVN, inferior vestibular neurons; MVN, medial vestibular nuclei; PC, posterior crista; S, saccule; sVN, superior vestibular neurons; U, utricle; Vmn, trigeminal motoneurons; VIII, vestibular projections. Modified after [3,23,67,123].
In contrast to the topology of the cochlear nuclei [11,128], the central vestibular neurons have an incomplete central segregation (Figure 3) that shows both segregation and overlap from different vestibular neurons [3,123,134]. Lateral line central projections can be segregated in certain vertebrates but show an overlap in other vertebrates [3,23]. For electroreception, multiple central topological projections in certain bony fish [27,135] show an overlap in lampreys and salamanders (Figure 3 [23,109]). The vestibular, lateral line, electroreception and cochlea independently reach hair cells that form prior to neurons [23,136], consistent with the same pattern of neurons that develop first, followed by the central axon to the brainstem, and later followed by the hair cell innervation [3,109,134,137]. This is obvious in cases where hair cells are not formed, such as in Atoh1 null mice, which show a near-normal central projection [131,138]. A similar central projection forms after the loss of hair cells in Pou4f1 null mice [139]. Loss of formation of a specific set of hair cells is demonstrated in the posterior canal that projects normally, despite the absence of Fgf10 [63], which degenerates later.
In summary, the neurons of the ear, lateral line and electroreception are generated by a set of genes that act downstream of Neurog1 to initiate the cell cycle. Neurons develop independently of central axons and reach innervate the hair cells shortly after proliferation. Segregation of central projections can be topologically organized in the auditory central projection of most tetrapods, and present two lateral line neurons that segregated in many vertebrates. Some central topology found in some, but not all, lateral line and electroreceptors, show an incomplete segregation for the vestibular neurons.
This entry is adapted from the peer-reviewed paper 10.3390/d13080364
This entry is offline, you can click here to edit this entry!
