Chronic pain is an unpleasant sensory and emotional experience that persists or recurs more than three months and may extend beyond the expected time of healing. Chronic pain occurs as a part of symptoms due to an underlying medical condition or remains despite successful treatment of the condition that originally caused it.
- chronic pain
- nociceptive pain
- neuropathic pain
- nociplastic pain
- psychogenic pain
- neuroinflammation
- kynurenine
1. Introduction
Chronic pain occurs as a part of symptoms due to an underlying medical condition or remains despite successful treatment of the condition that originally caused it [1]. Chronic pain frequently becomes the sole or predominant clinical complaint [2]. The prevalence of chronic pain estimates as much as 20%, and the incidence reaches about 10% every year of the world adult population [3]. Nearly 10% of individuals with chronic pain was found to suffer from moderate to severe debilitating pain [4]. Furthermore, individuals with severe chronic pain are twice more likely to die of respiratory disease or heart disease than those with mild pain or without pain [3]. The Global Burden of Disease Research ranked low back pain and migraine first and second place of Years Lived with Disability (YLD), respectively, and thus, chronic pain imposes a substantial socioeconomic burden directly and indirectly on society [5].
The International Classification of Diseases, Eleventh Revision (ICD-11), classifies chronic pain into primary and secondary. Primary chronic pain is fibromyalgia or low-back pain; the secondary chronic pain occurs secondary to an underlying medical condition subcategorizing into cancer-related, post-trauma, neuropathic, headache and orofacial, visceral, and musculoskeletal pain. ICD-11 offers minimal options for recording psychological or social factors in chronic pain [6]. Meanwhile, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) recognizes chronic pain in the diagnosis of somatic symptom disorder (SSD), having replaced pain disorder, a condition with chronic pain due to psychological factors [7]. SSD is caused by somatosensory amplification, which is associated with fibromyalgia [8]. The trend toward a neurological explanation obviously discounts cognitive, emotional, and social dimensions in the pathomechanism of chronic pain. Hyperalgesia is a condition of abnormally increased sensitivity to pain caused by injury to tissues or nerves. Nociceptive sensation is also caused by exposure to opioids used for pain treatment, which paradoxically makes individuals more sensitive to certain stimuli. Hyperalgesia is a challenging issue for pain specialists who treat patients at terminal care [9]. Chronic pain is often elicited by stimuli that previously did not provoke discomfort sensation. It is called allodynia. Allodynia is commonly observed in patients with neuropathies, fibromyalgia, migraine, complex regional pain syndrome, and postherpetic neuralgia [10]. Chronic pain may proceed to clinical conditions accompanied often by mood alterations, such as depression, anxiety, anger, cognitive disturbance including memory impairment, sleep disturbances, fatigue, loss of libido, and/or disability, called chronic pain syndrome (CPS). CPS appears to be linked to the dysfunction of the hypothalamic–pituitary–adrenal axis and the central nervous system (CNS), but exact mechanisms remain unknown [11].
Neuroinflammation has been intricately linked to the pathogenesis of chronic pain. Chronic pain was proposed to be caused by the disturbance of peripheral nociception, neuropathy in the somatosensory system, motor system, central and peripheral nociplasticity, and/or psychosocial system [12]. Increasing evidence suggests that chronic inflammation is strongly tied to aberration in each mechanism of chronic pain. Furthermore, the tryptophan (TRP)–kynurenine (KYN) pathway and its metabolites were observed to play an important role in neuroinflammation and chronic pain [13].
2. The Pain Pathway, Mechanisms, Neuroinflammation, and Tryptophan Metabolism
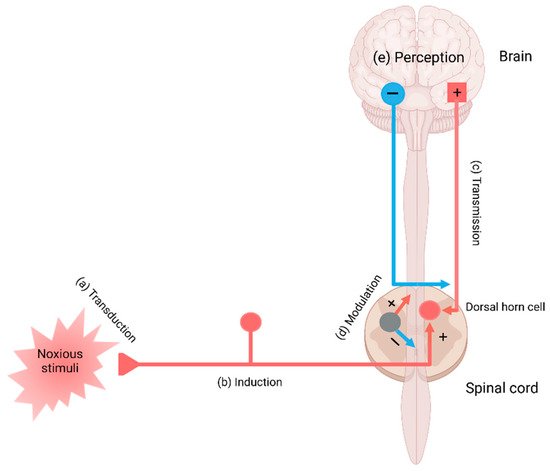
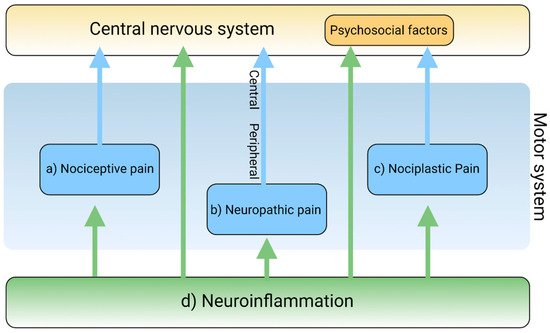
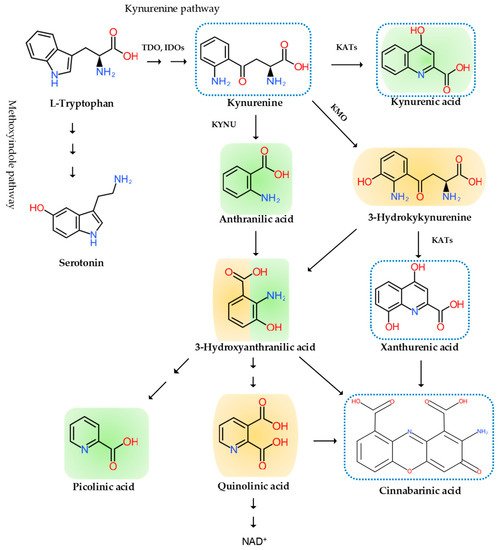
3. Conclusions and Future Perspective
Chronic pain arises through a complex pathogenic process involving more components and developing into the pain continuum. Central sensitization, peripheral sensitization, and somatization are pathogenic processes of pain development in the pain continuum spanning components of the pain pathway and the pain mechanism, which is hardly understood without the presence of the cortical perception. The nociplastic mechanism of pain attempts to delineate pain without relevant cause or lesions of the somatosensory nervous system, such as altered perception of nociception. Chronic pain presented in fibromyalgia syndrome, chronic back pain, and complex regional pain syndrome is best understood in the framework of pain perception, including cognitive, emotional, and social components. Chronic pain experienced in psychiatric conditions, in particular, is not fully explainable in the view of the nociplastic pain mechanism. Pain sensation is developed through complex interactions with higher cortical centers governing mood, emotion, and cognition.
More and more emerging findings shed light on the relationship between psychiatric symptoms and networks of the brain centers in neuropsychiatric disorders [38][39][40]. Stimulus-evoked functional magnetic resonance imaging (fMRI), task-free fMRI, and perfusion MRI revealed that chronic pains arise from pre-existing vulnerabilities and sustained abnormal input [41]. Neuroimaging techniques, including fMRI and positron emission tomography, may open the gate to understanding underlying mechanisms in signaling to the third-order neurons to the cortex in chronic pain sensation [42][43][44]. Pain relief can be achieved through accompanying symptoms such as cognition, mood, and sleep by pharmacotherapy and/or psychotherapy [42][45][46][47]. Therefore, psychogenic components of pain play an essential role in understanding the pathomechanism of chronic pain unless the nociplastic pain mechanism can sufficiently elucidate the reciprocal interaction with third-order neurons in the pathogenesis of chronic pain.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9080897
References
- Medicina. Special Issue “Chronic Pain Management”. Available online: https://www.mdpi.com/journal/medicina/special_issues/chronic_pain_management (accessed on 18 December 2020).
- Mäntyselkä, P.; Kumpusalo, E.; Ahonen, R.; Kumpusalo, A.; Kauhanen, J.; Viinamäki, H.; Halonen, P.; Takala, J. Pain as a reason to visit the doctor: A study in Finnish primary health care. Pain 2001, 89, 175–180.
- Mills, S.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283.
- Johnson, M.I. The Landscape of Chronic Pain: Broader Perspectives. Medicina 2019, 55, 182.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Available online: https://doi.org/10.1176/appi.books.9780890425596 (accessed on 27 December 2020).
- Ciaramella, A.; Silvestri, S.; Pozzolini, V.; Federici, M.; Carli, G. A retrospective observational study comparing somatosensory amplification in fibromyalgia, chronic pain, psychiatric disorders and healthy subjects. Scand. J. Pain 2020.
- Rivat, C.; Ballantyne, J. The dark side of opioids in pain management: Basic science explains clinical observation. Pain Rep. 2016, 1, e570.
- He, Y.; Kim, P.Y. Allodynia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537129/ (accessed on 27 December 2020).
- Yasaei, R.; Peterson, E.; Saadabadi, A. Chronic Pain Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470523/ (accessed on 18 December 2020).
- Chimenti, R.L.; Frey-Law, L.A.; Sluka, K.A. A Mechanism-Based Approach to Physical Therapist Management of Pain. Phys. Ther. 2018, 98, 302–314.
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The Role of the Kynurenine Signaling Pathway in Different Chronic Pain Conditions and Potential Use of Therapeutic Agents. Int. J. Mol. Sci. 2020, 21, 6045.
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56.
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Franco, N.F.; Ng, M.L.; Pai, S.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front. Immunolog. 2016, 7, 246.
- Ong, W.Y.; Stohler, C.S.; Herr, D.R. Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 2019, 56, 1137–1166.
- Trouvin, A.P.; Perrot, S. New concepts of pain. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101415.
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19.
- Gonçalves dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial Cytokines in Pain States. Front Immunol. 2020, 10, 3061.
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577.
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139.
- Misiak, B.; Frydecka, D.; Stanczykiewicz, B.; Samochowiec, J. Editorial: Peripheral Markers of Immune Response in Major Psychiatric Disorders: Where Are We Now and Where Do We Want to Be? Front. Psychiatry 2019, 10, 5.
- Verlaet, A.A.J.; Maasakkers, C.M.; Hermans, N.; Savelkoul, H.F.J. Rationale for Dietary Antioxidant Treatment of ADHD. Nutrients 2018, 10, 405.
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155.
- Encyclopedia. The Tryptophan-Kynurenine Metabolic Pathway. Available online: https://encyclopedia.pub/8633 (accessed on 13 April 2021).
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734.
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564.
- Dezsi, L.; Tuka, B.; Martos, D.; Vecsei, L. Alzheimer’s disease, astrocytes and kynurenines. Curr. Alzheimer Res. 2015, 12, 462–480.
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338.
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822.
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum Kynurenines Correlate with Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabilit. Neural Repair 2020, 34, 936–944.
- Tanaka, M.; Török, N.; Vécsei, L. Novel Pharmaceutical Approaches in Dementia. In NeuroPsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer: Cham, Switzerland, 2021; Available online: https://doi.org/10.1007/978-3-319-56015-1_444-1 (accessed on 1 June 2021).
- Ulivieri, M.; Wierońska, J.M.; Lionetto, L.; Martinello, K.; Cieslik, P.; Chocyk, A.; Curto, M.; Di Menna, L.; Iacovelli, L.; Traficante, A.; et al. The Trace Kynurenine, Cinnabarinic Acid, Displays Potent Antipsychotic-Like Activity in Mice and Its Levels Are Reduced in the Prefrontal Cortex of Individuals Affected by Schizophrenia. Schizophr. Bull. 2020, 46, 1471–1481.
- Tanaka, M.; Vécsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406.
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431.
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2,3-Dioxygenase 1 Influenced the Age Onset of Parkinson’s Disease. Preprints 2020.
- Tanaka, M.; Vécsei, L. Monitoring the Kynurenine System in Neurodegenerative and Psychiatric Illnesses: Concentrations, Ratios, or What Else? Adv. Clin. Exp. Med 2021, 30. in press.
- Kowalska, K.; Krzywoszański, Ł.; Droś, J.; Pasińska, P.; Wilk, A.; Klimkowicz-Mrowiec, A. Early Depression Independently of Other Neuropsychiatric Conditions, Influences Disability and Mortality after Stroke (Research Study—Part of PROPOLIS Study). Biomedicines 2020, 8, 509.
- Cantón-Habas, V.; Rich-Ruiz, M.; Romero-Saldaña, M.; Carrera-González, M.P. Depression as a Risk Factor for Dementia and Alzheimer’s Disease. Biomedicines 2020, 8, 457.
- Park, S.; Bak, A.; Kim, S.; Nam, Y.; Kim, H.; Yoo, D.-H.; Moon, M. Animal-Assisted and Pet-Robot Interventions for Ameliorating Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Biomedicines 2020, 8, 150.
- Davis, K.D.; Moayedi, M. Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 2013, 8, 518–534.
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340.
- Kim, J.; Kim, Y.-K. Crosstalk between Depression and Dementia with Resting-State fMRI Studies and Its Relationship with Cognitive Functioning. Biomedicines 2021, 9, 82.
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric Neural Networks and Precision Therapeutics by Machine Learning. Biomedicines 2021, 9, 403.
- Bannister, K.; Kucharczyk, M.; Dickenson, A.H. Hopes for the future of pain control. Pain Ther. 2017, 6, 117–128.
- Kordestani-Moghadam, P.; Assari, S.; Nouriyengejeh, S.; Mohammadipour, F.; Pourabbasi, A. Cognitive impairments and associated structural brain changes in metabolic syndrome and implications of neurocognitive intervention. J. Obes. Metab. Syndr. 2021, 29, 174–179.
- Kordestani-Moghadam, P.; Nasehi, M.; Vaseghi, S.; Khodagholi, F.; Zarrindast, M.R. The role of sleep disturbances in depressive-like behavior with emphasis on α-ketoglutarate dehydrogenase activity in rats. Physiol. Behav. 2020, 224, 113023.
