Cobalt oxide (Co3O4) is known to follow the spinel structure as (Co2+)[Co23+O4. The high spin Co2+occupies the interstitial sites of tetrahedral (8a) whereas low spin Co3+are known to occupy the interstitial sites of octahedral (16d) of the close-packed face-centered cubic lattice of CoO.Co2O3. The p-type conductivity of the material (CoO.Co2O3) is known to originate from the vacancies of Co in the crystal lattices or/and excess oxygen at interstitial sites. Furthermore, 1D nanostructures of Co3O4 have been investigated over the past decades as an active material for chemical analytes detection owing to its superior catalytic effect together with its excellent stability. This article discusses the state-of-the-art of growth and characterization of Co3O4 1D nanostructures and their functional characterization as chemical/gas sensors.
- cobalt oxide
- 1D nano structures
- chemical gas sensor
1. Introduction
A chemical sensor is defined as a device that transforms chemical information into an analytically useful signal [1]. Generally, chemical information originates from the chemical reaction of the analyte with the active part of the sensor that induces a variation of the properties of the sensor. However, not only the chemical reaction but also physical processes (adsorption-desorption), mechanisms of electrical conductivity of sensitive material, etc., contribute to the overall sensitivity of the sensor [1]. Chemical/gas sensors are identified as excellent candidates for the detection and quantification of chemical/gas compounds due to their direct electronic interface, fast response, high sensitivity, and low cost of production [1],[2],[3],[4],[5],[6],[7],[8],[9]]. In general, chemical/gas sensors are based on oxidation or reduction of the active material by the surrounding atmosphere. Numerous types of chemical/gas-sensing materials including metal oxide semiconductors [2],[3],[4],[5],[6], conducting polymers [7], conducting polymer composites [8],[9], carbon nanomaterials [10], metal oxide/polymer composites [11],[12], and various transduction mechanisms have been reported over the past decades.
Metal oxide (MOX) semiconductors may be divided in two different groups depending on their majority carriers. For instance, in n-type MOX semiconductors, such as ZnO, In 2O 3, Fe 3O 2, TiO 2, WO 3, SnO 2, the majority carriers are electrons, while in p-type, NiO, Co 3O 4, Cr 2O 3, Mn 3O 4, CuO, the majority carriers are holes [13],[14],[15]. In contrast to n-type MOX gas sensors, p-type MOX ones are less studied, and the reported works are still in a premature stage of development. Figure 1 shows the results of p-type and n-type MOX gas sensors in Web of Knowledge on 30 June 2021 with the keyword “metal oxide gas sensor.” Only 13% of articles report on the p-type MOX gas sensors out of a total of 24,234 articles concerning MOX gas sensors. Irrespective of the number of reported works, p-type metal oxides have shown excellent performances in chemical/gas sensing owing to the majority of charge carriers, their conduction paths, resistance to humidity influence on sensing performances, superior catalyst properties, long-term stability, and lower open circuit resistance in contrast to n-type MOX gas sensors [16]. Furthermore, oxygen is excellently adsorbed at p-type MOX surface at low temperature compared to n-type. Thereby p-type MOX gas sensors could potentially be used for the sensing of volatile organic compounds (VOCs) since they are highly oxidized by adsorbed oxygen at the surface compared to surface lattice oxygen [17],[18],[19]. Moreover, the excellent gas-sensing performances at low working temperatures in p-type MOX gas sensors have paved the interest for the detection and quantification of VOCs [16]. Thereby, the number of scientific research works was gradually increased on p-type MOX as shown in Figure 2 .
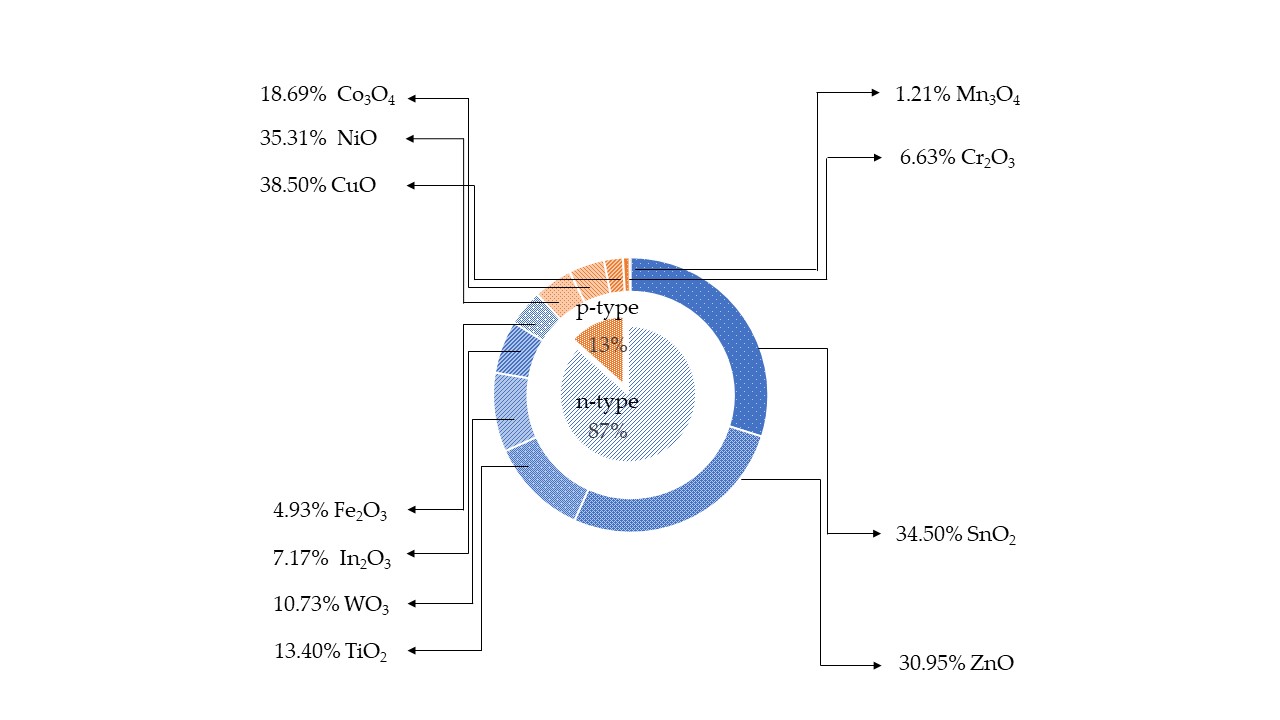
Figure 1. The reported scientific research on n-type and p-type metal oxide gas sensors in Web of Knowledge on 30 June 2021.
Consequently, scientific works on Co3O4 gas-sensing applications have been gradually increased ( Figure 2 ) due to tremendous sensitivity, response/recovery, and stability even at lower and elevated temperatures, superior electrical and chemical properties together with their abundance [20],[21]. Similarly, various nanostructures of Co3O4, nanoparticles [22],[23], nanowires [24],[25],[26],[27],[28],[29], nanorods[30],[31], nanosheets [32],[33], nanocubes [34], nanoneedles [35], hollow microspheres [36],[37],[38], urchin-like structures [39],[40],[41], have been reported in the literature for the detection of CH3OH, C2H5OH, HCHO, CH4, CH 3COCH3, C6H6, C6H5CH3, C6H5(CH3)2, CO.
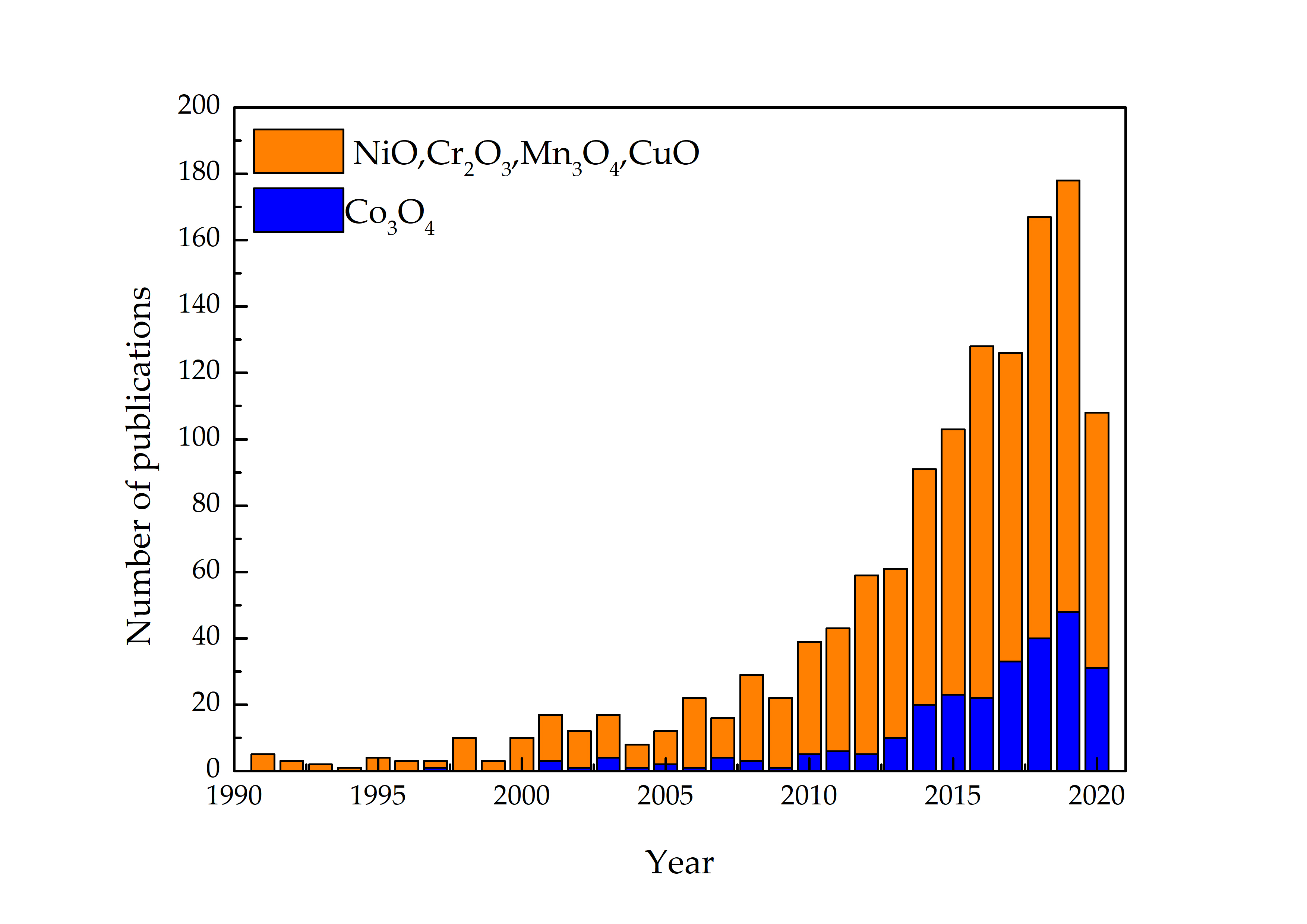
Figure 2. The advancement of p-type MOX gas sensors by yearly from the search on Web of Knowledge on 30 June 2021.
However, the significant improvements in the application of one dimensional (1D) nanostructures of Co 3O 4, on chemical/gas sensing have been attributed to the superior adsorption—desorption of chemical compounds which results in an outstanding response and reliability for chemicals/gases detection [42],[43]. Additionally, the higher surface energy, crystalline quality, large intrinsic resistance modulation, number of reactive sites, and large surface to volume ratio of 1D nanostructures have significantly improved the gas-sensing performances [14],[44].
2. Material and Sensing
The key parameters that can be useful when discussing chemical/gas sensors are response, selectivity, response time, recovery time, stability, gas detection limit, and operating temperature. A comprehensive but brief understanding on each parameter is useful prior to the detailed discussion on chemical/gas sensors.
Response may be described as the fraction of the resistance on the sensing material when interacting with the analyte gas (Rg ) and the one in air (Ra ). However, response vastly relies on the crystallite size, porosity, operating temperature, and film thickness. Selectivity determines the ability to identify the target gas in a mixture of gases and can be tuned with the operating temperature. However, MOX chemical/gas sensors may respond in similar manners toward different gas molecules [45]. Hence the selectivity is one of the paramount features when describing the sensing performances. Stability is the ability of the active material to keep its properties, such as electrical resistance in the case of conductometric sensors, constant over time. Response time is an evaluation of the dynamics of the sensor to achieve a stable value of the monitored sensor parameter, for example, the time to accomplish 90% of the final resistance of the sensor when interacting with the gas. Usually, the electrical resistance of the conductometric chemical/gas sensors changes when interacting with the analytes molecules as shown in Table 1. In general, a dynamic response curve shows the resistance/conductance variation of the sensor with time. While the recovery time may be calculated as the time interval necessary to get back to 90% of the resistance value in the air before the gas introduction as the airflow is restored. These parameters can be calculated from the dynamic response plot as shown in Figure 3. The detection limit is the least concentration of analyte gas that may be detected by the sensor. Lastly, working temperature is the temperature where the sensor is operating.
Table 1. Variation of the resistance in MOX upon the interaction of reducing or oxidizing gases.
|
Metal Oxide |
Reducing Gases |
Oxidizing Gases |
|
p-type |
Resistance increase |
Resistance decrease |
|
n-type |
Resistance decrease |
Resistance increase |
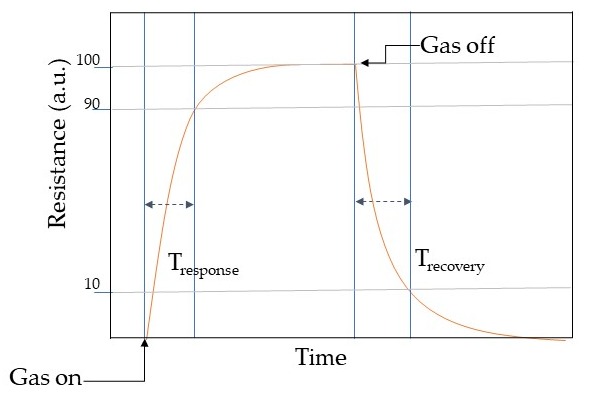
Figure 3. Schematic illustration of a dynamic response curve of a conductometric chemical/gas sensor.
In general, bottom-up or top-down approaches are used for the growth of nanostructures. The experimental setup for bottom-up approaches may be low cost producing high crystallinity and purity nanostructures, but their alignment may be extremely difficult. Top-down approaches suffer from long preparation times, while they easily form structures directly on flat substrates. Concerning the growth of Co3O4 nanostructure as chemical/gas sensors, several growth techniques such as hydrothermal, electrospinning, and solvothermal were widely employed.
3. Overview of Reported 1 D Nano-Structured Co3O4 Gas Sensors
3.1 Sensing Toward Ethanol (C2H5OH)
Ethanol is a VOC that is vastly present in the daily life of human beings in means of food, beverages, fuel-processing, pharmaceutical, as well as in many laboratories and industries for diverse research and applications [47]. However, long-term exposure to C2H5OH has been identified as the main reason for serious health effects such as lethargy, irritation to skin and eye, difficulty breathing, coma, liver damage, and intoxication. Moreover, C2H5OH as an alcoholic drink has been identified as one of the root causes for rising traffic accidents around the world [48]. For this reason, almost all countries have legalized the consumption limitation of C2H5OH. For example, in Italy, the highest ethanol level that is allowed in the breath of drivers is 130 ppm (0.05% in the blood) while in the USA, 208 ppm (0.08% in the blood) [49]. Hence, detection and quantification of C2H5OH in the environment as well as in human breath have become a necessity. Before discussing the C2H5OH-sensing applications it would be interesting to understand its sensing mechanism at MOXs surface. When ethanol is interacting with the near-surface irrespective of the type of MOXs sensor, these molecules are chemisorbed on the MOXs surfaces. Afterward, chemisorbed ethanol molecules start to react with the adsorbed oxygen species resulting in the formation of H2O and CO2. Hence the trapped electrons are liberated back to the MOXs altering the sensor resistance. The chemical reaction between C2H5OH and adsorbed oxygen species on the near-surface of the MOXs is described as in (1–2) regardless of the type of the MOXs [49].
C2H5OH (ads) + O¯ (ads) → CH3CHO (ads) + H2O + e− (1)
CH3CHO (ads) + 5O− (ads) → 2CO2 +2H2O + 5e− (2)
Electrospinning has been employed for the preparation of nanofibers (NFs) for the detection of C2H5OH by Yoon et al.[50]. The sensors have shown superior sensitivity (Rg /Ra = 51.2) toward 100 ppm of C2H5OH compared to CO, C3H8, and H2 at 301 °C before decreasing to 19.2 at 336 °C. While the recovery time was decreasing as the operating temperature increases due to the thermal boosting of oxygen ionization.
3.2 Sensing toward Acetone (C3H6O)
A superior sensitive and selective performance toward acetone has been reported by Choi et al. on Co3O4 NFs composite with Ir NPs and graphene oxide (GO) sheets in 2014 [66]. Co3O4 NFs, have been grown by electrospinning technique while the GO sheets as well as Ir NPs were grown by the polyol method [68]. The composites NFs (1 wt% Ir-GO-Co3O4) were able to detect acetone even at 120 ppb in highly humid ambient conditions (90% RH) demonstrating great potentialities for acetone analysis in human breath [66]. In addition, the sensors have shown excellent selectivity toward acetone other than the usual breath biomarkers; pentane, NO2, NO, NH3, and CO. One of the reasons for these excellent performances is the combined catalytic effect of GO sheets and Ir NPs. A second one is the hole accumulation layer thinning on the surface of Co3O4 NFs due to Ir NPs together with the effective electronic sensitization by the GO sheets. In addition, the rapid transfer of the carrier to the sensing electrodes, due to the highly conductive GO sheets, contributed to improving the performances.
Another interesting morphology for chemical compounds detection is one of the hierarchical nanofibers (HNF). Its effective surface area is outstanding and there is a smooth transfer of carriers through nanofibers without additional barriers, due to the connection of the nanosheet-like structures in the nanofiber [51]. In 2019 Cao et al. have demonstrated C3H6O sensing with HNFs at an operating temperature of 190 °C. The grown HNFs comprised many nanosheets with a smooth surface almost perpendicular to the nanofiber’s surfaces (thickness of 20–40 nm) [51]. However, Zhang et al. have reported the possibility of detecting C3H 6O at an operating temperature of 75 °C with Co3O4 NWs assembled on hollow carbon spheres (Co3O4 NW-HCS) [23]. The enhanced sensing performances at low operating temperatures have been attributed to the interaction between hollow carbon spheres and short Co3O4 nanowires. Moreover, the unique porous cavity structure of hollow carbon spheres contributes to the excellent sensing performances by paving a higher number of available sites for easy adsorption and desorption of C3H6O molecules.
3.3 Sensing toward Carbon Monoxide (CO)
In 2010 Patil et al. have demonstrated Co3O4 NRs sensing of CO with a response of 6.5 to 50 ppm CO at 250 °C [15]. The NRs sensors have demonstrated a higher response compared to the commercially available NP sensors due to the stronger bonding between nanoparticles in the NRs beside the higher surface-to-volume ratio. Additionally, the fabricated sensors have shown superior selectivity compared to H 2, LPG, CO2, and ethanol.
However, CO sensing at a low working temperature (100 °C) has been reported by Dou et al. in 2014 [16]. The NFs sensors demonstrated a higher response (~13.5 (Rg/Ra) toward 50 ppm CO. Moreover, the detection of 5 ppm at 100 °C has been reported by the Busacca et al. on NFs Co3O4 sensors in the same year [62]. The excellent CO-sensing response of 2.4 (Rg/Ra) together with a response time of 14 s and recovery time of 36 s of NFs was attributed to the higher effective surface area as well as the higher number of oxygen vacancies or defects on the NFs surface. Additionally, the lower crystallite size (about 15 nm) has been identified as one of the potential factors that could enhance sensor performances. However, the sensor response was saturated at a concentration of 20 ppm at 100 °C owing to the formation of carbonates species at a low temperature that deactivates the action of Co3O4 catalysts in CO oxidation.
The sensing mechanism can be understood as follows.
|
CO(g) → CO(ads) |
(3) |
|
CO(ads) + O− → CO2 + e− |
(4) |
|
CO + OH−(ads) ↔ HCO2¯ ↔ H+(ads) + CO2 + 2 e− |
(5) |
|
CO + H2O → CO2 + H2 |
(6) |
4. Conclusions and Outlook
Usually, Co3O4 is a p-type MOX semiconductor with excellent catalytic properties together with higher stability in chemical/gas testing even in humid conditions. Among the different growth techniques of growing the Co3O4 nano structures, hydrothermal, solvothermal, and electrospinning techniques have been vastly employed owing to higher yield, simplicity, and scalability. The excellent catalytic effect of the Co3O4 has been identified as one of the root causes for the achievement of exceptional sensing performance turning to operate at elevated working temperatures as well as the long-term stability. However, Co3O41D nano structures are still capable of detecting few gases such as CO, H2S, NH3, and some VOCs such as C2H5OH , C3H6O, HCHO, C4H 10O, and C3H9O 3P. Among these gases and VOCs, Co3O41D nano structures are highly selective toward C2H5OH and C3H6O. Besides, the sensing performances are highly dependent on the morphology where rougher topology along with the unique structure of Co3O4 NR arrays to a large extent could be the best choice for the superior gas-sensing performances. Nonetheless, low working temperature regimes can be proposed for the chemical/gas sensors based on Co3O4 ID nano structures owing to its excellent catalytic properties. However, a complete and well-controlled characterization of operating temperature on the key factors such as response time, recovery time of the Co3O4 1D chemical/gas sensors would not be far away because of rapid development of the new technologies adapted to nanomaterials.
In conclusion, a reliable material growth process and cheaper production cost of the devices must be achieved for large-scale production of both active material and transducer. Unfortunately, most of the reported sensors in this review article do not provide a significant indication for the possibility of large-scale production. However, various fabrication and characterization strategies have been developed over the past decade to demonstrate the possible application of Co3O4 1D nanostructures as reliable and cheaper chemical/gas sensors. As long as an appropriate optimization of the growth technologies will allow the controlled and reliable production of Co3O4 1D nanostructures together with improvement in the sensor’s performances, the large-scale production and their integration in commercial gas-sensing devices will certainly follow owing to the day by day improvements and progresses in materials research.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors9080197
References
- Hulanicki, A.; Glab, S.; Ingman, F.; Chemical Sensors: Definitions and Classification. Pure Appl. Chem 1991, 63, 1247, 10.1351/pac199163091247.
- Qiao, X.; Xu, Y.; Yang, K.; Ma, J.; Li, C.; Wang, H.; Jia, L.; Mo Doped BiVO4 Gas Sensor with High Sensitivity and Selectivity Towards H2S. Chem. Eng. Sci. 2020, 395, 125144, 10.1016/j.cej.2020.125144.
- Choi, K.; Kim, H.; Kim, K.; Liu, D.; Cao, G.; Lee, J.; C2H5OH Sensing Characteristics of Various Co3O4 Nanostructures Prepared by Solvothermal Reaction. Sens. Actuators B Chem. 2010, 146, 183, 10.1016/j.snb.2010.02.050.
- 4. Aqeel, T.; Galstyan, V.; Comini, E.; Mesoporous Polycrystalline SnO2 Framework Synthesized by Direct Soft Templating Method for Highly Selective Detection of NO2.. Nanotechnology 2019, 31, 105502, 10.1088/1361-6528/ab5a1e.
- Comini, E.; Metal Oxide Nano-Crystals for Gas Sensing. Anal. Chim. Acta 2006, 568, 28, 10.1016/j.aca.2005.10.069.
- Comini, E.; Yubao, L.; Brando, Y.; Sberveglieri, G.; Gas Sensing Properties of MoO3 Nanorods to CO and CH3OH. Chem. Phys. Lett. 2005, 407, 368, 10.1016/j.cplett.2005.03.116.
- Wong, Y.; Ang, B.; Haseeb, A.; Baharuddin, A.; Wong, Y.; Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2019, 167, 037503, 10.1149/2.0032003jes.
- Husain, A.; Ahmad, S.; Mohammad, F; Synthesis, Characterisation and Ethanol Sensing Application of Polythiophene/Graphene Nanocomposite. Mater. Chem. Phys. 2020, 239, 122324, 10.1016/j.matchemphys.2019.122324.
- Guo, Z.; Liao, N.; Zhang, M.; Feng, A.; Enhanced Gas Sensing Performance of Polyaniline Incorporated with Graphene: A First-Principles Study.. Phys. Lett. A 2019, 383, 2751, 10.1016/j.physleta.2019.03.045.
- 10. Reddeppa, M.; Chandrakalavathi, T.; Park, B.; Murali, G.; Siranjeevi, R.; Nagaraju, G.; Su Yu, J.; Jayalakshmi, R.; Kim, S.; Kim, M.; et al. UV-Light Enhanced CO Gas Sensors Based on Ingan Nanorods Decorated With P-Phenylenediamine-Graphene Oxide Com-posite. Sens. Actuators B Chem. 2020, 307, 127649, 10.1016/j.snb.2019.127649.
- Harraz, F.; Faisal, M.; Jalalah, M.; Almadiy, A.; Al-Sayari, S.; Al-Assiri, M.; Conducting Polythiophene/Α-Fe2O3 Nanocomposite for Efficient Methanol Electrochemical Sensor. Appl. Surf. Sci. 2020, 508, 145226, 10.1016/j.apsusc.2019.145226.
- 12. Choi, J.; Hwang, I.; Kim, S.; Park, J.; Park, S.; Jeong, U.; Kang, Y.; Lee, J.; Design of Selective Gas Sensors Using Electrospun Pd-Doped SnO2 Hollow Nanofibers. . Sens. Actuators B Chem 2010, 150, 191, 10.1016/j.snb.2010.07.013.
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R.; Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088, 10.3390/s100302088.
- Kim, H.; Lee, J.; Highly Sensitive and Selective Gas Sensors Using P-Type Oxide Semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607, 10.1016/j.snb.2013.11.005.
- Patil, D.; Patil, P.; Subramanian, V.; Joy, P.; Potdar, H.; Highly Sensitive and Fast Responding CO Sensor Based on Co3O4 Nanorods. Talanta 2010, 81, 37, 10.1016/j.talanta.2009.11.034.
- Dou, Z.; Cao, C.; Chen, Y.; Song, W.; Fabrication of Porous Co3O4 nanowires with High CO Sensing Performance at A Low Operating Temperature. Chem. Commun. 2014, 50, 14889, 10.1039/c4cc05498a.
- 17. Zhang, X.; Wang, J.; Xuan, L.; Zhu, Z.; Pan, Q.; Shi, K.; Zhang, G.; Novel Co3O4 Nanocrystalline Chain Material as A High Performance Gas Sensor at Room Temperature. J. Alloys Compd. 2018, 768, 190, 10.1016/j.jallcom.2018.07.240.
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Tan, W.; Lin, Z.; Huang, X.; Synthesis of Hollow and Hollowed-Out Co3O4 Microspheres Assembled by Porous Ultrathin Nanosheets For Ethanol Gas Sensors: Responding and Recovering in One Second.. Sens. Actuators B Chem. 2017, 249, 44, 10.1016/j.snb.2017.04.063.
- Tan, W.; Tan, J.; Li, L.; Dun, M.; Huang, X.; Nanosheets-Assembled Hollowed-Out Hierarchical Co3O4 Microrods For Fast Response/Recovery Gas Sensor.. Sens. Actuators B Chem. 2017, 249, 66, 10.1016/j.snb.2017.04.068.
- 20. Yuan, Y.; Wang, Y.; He, X.; Chen, M.; Liu, J.; Liu, B.; Zhao, H.; Liu, S.; Yang, H; . Increasing Gas Sensitivity of Co3O4 Octahedra by Tuning Co-Co3O4 (111) Surface Structure and Sensing Mechanism of 3-Coordinated Co Atom as An Active Center. . J. Mater. Sci.: Mater. Electron. 2020, 31, 8852, 10.1007/s10854-020-03420-9.
- Zhou, Q.; Zeng, W.; Shape Control of Co3O4 Micro-Structures for High-Performance Gas Sensor. . Phys. E Low Dimens. Syst. Nanostruct. 2018, 95, 121, 10.1016/j.physe.2017.09.009.
- Xu, K.; Yu, X.; Zhao, W.; Zeng, W.; Density-Dependent of Gas-Sensing Properties of Co3O4 Nanowire Arrays. . Phys. E Low Dimens. Syst. Nanostruct. 2020, 118, 113956, 10.1016/j.physe.2020.113956.
- Kozlovskiy, A.; Zdorovets, M.; The Study of The Structural Characteristics and Catalytic Activity of Co/CoCo2O4 Nanowires. Compos. B. Eng. 2020, 191, 107968, 10.1016/j.compositesb.2020.107968.
- Nguyen, H.; El-Safty, S.; Meso and Macroporous Co3O4 Nanorods for Effective VOC Gas Sensors. . J. Phys. Chem. C 2011, 115, 8466, 10.1021/jp1116189.
- Wen, Z.; Zhu, L.; Mei, W.; Hu, L.; Li, Y.; Sun, L.; Cai, H.; Ye, Z; Rhombus-Shaped Co3O4 Nanorod Arrays for High-Performance Gas Sensor. Sens. Actuators B Chem. 2013, 186, 172, 10.1016/j.snb.2013.05.093.
- Wang, S.; Cao, J.; Cui, W.; Fan, L.; Li, X.; Li, D.; Zhang, T.; One-Dimensional Porous Co3O4 Rectangular Rods for Enhanced Acetone Gas Sensing Properties. Sens. Actuators B Chem 2019, 297, 126746, 10.1016/j.snb.2019.126746.
- Wang, L.; Deng, J.; Lou, Z.; Zhang, T.; Nanoparticles-Assembled Co3O4 Nanorods P-Type Nanomaterials: One-Pot Synthesis and Toluene-Sensing Properties. . Sens. Actuators B Chem. 2014, 201, 1, 10.1016/j.snb.2014.04.074.
- Vetter, S.; Haffer, S.; Wagner, T.; Tiemann, M; Nanostructured Co3O4 as a CO Gas Sensor: Temperature-Dependent Behavior. Sens. Actuators B Chem 2015, 206, 133, 10.1016/j.snb.2014.09.025.
- Jiang, R.; Jia, L.; Guo, X.; Zhao, Z.; Du, J.; Wang, X.; Wang, P.; Xing, F.; Dimethyl Sulfoxide-Assisted Hydrothermal Synthesis of Co3O4-Based Nanorods For Selective and Sensitive Diethyl Ether Sensing. Sens. Actuators B Chem. 2019, 290, 275, 10.1016/j.snb.2019.03.136.
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y.; Highly Sensitive VOC Gas Sensor Using P-Type Mesoporous Co3O4 Nanosheets Prepared by A Facile Chemical Coprecipita-tion Method. Sens. Actuators B Chem. 2016, 233, 615, 10.1016/j.snb.2016.04.138.
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L.; Gas Sensors Based on Ultrathin Porous Co3O4 Nanosheets to Detect Acetone at Low Temperature. RSC Adv. 2015, 5, 59976, 10.1039/c5ra08536e.
- Lü, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L.; MOF-Templated Synthesis of Porous Co3O4 Concave Nanocubes with High Specific Surface Area and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 4186, 10.1021/am405858v.
- Wen, Z.; Zhu, L.; Li, Y.; Zhang, Z.; Ye, Z; Mesoporous Co3O4 Nanoneedle Arrays for High-Performance Gas Sensor12. Sens. Actuators B Chem. 2020, 263, 127215, 10.1016/j.snb.2014.06.124.
- Wen, Z.; Zhu, L.; Li, Y.; Zhang, Z.; Ye, Z.; Mesoporous Co3O4 Nanoneedle Arrays for High-Performance Gas Sensor. Sens. Actuators B Chem. 2014, 203, 873, 10.1016/j.snb.2014.06.124.
- Park, J.; Shen, X.; Wang, G.; Solvothermal Synthesis and Gas-Sensing Performance of Co3O4 Hollow Nanospheres. Sens. Actuators B Chem. 2009, 136, 467, 10.1016/j.snb.2008.11.041.
- Shen, S.; Xu, M.; Lin, D.; Pan, H.; The Growth of Urchin-Like Co3O4 Directly on Sensor Substrate and Its Gas Sensing Properties. Appl. Surf. Sci 2017, 396, 327, 10.1016/j.apsusc.2016.10.147.
- Li, C.; Yin, X.; Wang, T.; Zeng, H; Morphogenesis of Highly Uniform CoCo3 Submicrometer Crystals and Their Conversion to Mesoporous for Gas-Sensing Ap-plications. Chem. Mater. 2009, 21, 4984, 10.1021/cm902126w.
- Deng, S.; Chen, N.; Deng, D.; Li, Y.; Xing, X.; Wang, Y; Meso and Macroporous Coral-Like Co3O4 for VOCs Gas Sensor. Sensor. Ceram. Int. 2015, 41, 11004, 10.1016/j.ceramint.2015.05.045.
- Lee, J.; Gas Sensors Using Hierarchical and Hollow Oxide Nanostructures: Overview. Sens. Actuators B Chem 2009, 140, 319, Sens. Actuators B Chem.
- Xu, J.; Zhang, Y.; Chen, Y.; Xiang, Q.; Pan, Q.; Shi, L.; Uniform ZnO Nanorods Can be Used to Improve the Response of ZnO Gas Sensor. Mater. Sci. Eng. B 2008, 150, 55, 10.1016/j.mseb.2008.01.010.
- Xu, J.; Wang, D.; Qin, L.; Yu, W.; Pan, Q.; SnO2 Nanorods and Hollow Spheres: Controlled Synthesis and Gas Sensing Properties. Sens. Actuators B Chem 2009, 137, 490, 10.1016/j.snb.2009.01.011.
- Wang, L.; Song, S.Y.; Hong, B.; Xu, J.C.; Han, Y.B.; Jin, H.X.; Jin, D.F.; Li, J.; Yang, Y.T.; Peng, X.L.; et al. Highly improved toluene gas-sensing performance of mesoporous Co3O4 nanowires and physical mechanism. Mater. Res. Bull 2021, 140, 111329, 10.1016/j.materresbull.2021.111329.
- Qiao, X.; Ma, C.; Chang, X.; Li, X.; Li, K.; Zhu, L.; Xia, F.; Xue, Q; 3D radial Co3O4 nanorod cluster derived from cobalt-based layered hydroxide metal salt for enhanced trace acetone detec-tion. Sens. Actuators B Chem 2021, 324, 128926, 10.1016/ j.snb.2020.128926.
- Xu, J.; Cheng, J.; The Advances of Co3O4 as Gas Sensing Materials: A Review. J. Alloys Compd. 2016, 686, 753, 10.1016/j.jallcom.2016.06.086.
- Kaur, N.; Singh, M.; Moumen, A.; Duina, G.; Comini, E; 1D Titanium Dioxide: Achievements in Chemical Sensing. Materials 2020, 13, 2974, 10.3390/ma13132974.
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U; Modeling of Sensing and Transduction for P-Type Semiconducting Metal Oxide Based Gas Sensors. J. Electroceram 2009, 25, 11, 10.1007/s10832-009-9583-x.
- Feng, Q.; Li, X.; Wang, J.; Gaskov, A.; Reduced Graphene Oxide (RGO) Encapsulated Co3O4 Composite Nanofibers for Highly Selective Ammonia Sensors. Sensor actuat. B-chem 2016, 2222, 864, 10.1016/j.snb.2015.09.021.
- Thungon, P.; Kakoti, A.; Ngashangva, L.; Goswami, P; Advances in Developing Rapid, Reliable and Portable Detection Systems for Alcohol. Biosens. Bioelectron 2017, 97, 83, 10.1016/j.bios.2017.05.041.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.; Neri, G.; Highly Stable and Selective Ethanol Sensor Based on Α-Fe2O3 Nanoparticles Prepared by Pechini Sol–Gel Method. Ceram. Int. 2016, 42, 6136, 10.1016/j.ceramint.2015.12.176.
- Yoon, J.; Choi, J.; Lee, J.; . Design of a Highly Sensitive and Selective C2H5OH Sensor Using P-Type Co3O4 Nanofibers. Sens. Actuators B Chem. 2012, 161, 570, 10.1016/j.snb.2011.11.002.
- Cao, J.; Wang, S.; Zhang, H.; Zhang, T; Constructing One Dimensional Co3O4 Hierarchical Nanofibers as Efficient Sensing Materials for Rapid Acetone Gas Detec-tion. J. Alloys Compd 2019, 799, 513, 10.1016/j.jallcom.2019.05.356.
