Oral candidosis is the most common fungal infection that frequently occurs in patients debilitated by other diseases or conditions. No candidosis happens without a cause; hence oral candidosis has been branded as a disease of the diseased. Prior research has identified oral candidosis as a mark of systemic diseases, such as hematinic deficiency, diabetes mellitus, leukopenia, HIV/AIDS, malignancies, and carbohydrate-rich diet, drugs, or immunosuppressive conditions. An array of interaction between Candida and the host is dynamic and complex. Candida exhibits multifaceted strategies for growth, proliferation, evasion of host defenses, and survival within the host to induce fungal infection.
- oral candidosis
- systemic candidosis
- hematinic deficiency
- anemia
- immunosuppression
- cell-mediated immunity
1. Overview
Oral candidosis is the most common fungal infection that frequently occurs in patients debilitated by other diseases or conditions. No candidosis happens without a cause; hence oral candidosis has been branded as a disease of the diseased. Prior research has identified oral candidosis as a mark of systemic diseases, such as hematinic deficiency, diabetes mellitus, leukopenia, HIV/AIDS, malignancies, and carbohydrate-rich diet, drugs, or immunosuppressive conditions. An array of interaction between Candida and the host is dynamic and complex. Candida exhibits multifaceted strategies for growth, proliferation, evasion of host defenses, and survival within the host to induce fungal infection. Oral candidosis presents a variety of clinical forms, including pseudomembranous candidosis, erythematous candidosis, angular cheilitis, median rhomboid glossitis, cheilocandidosis, juxtavermillion candidosis, mucocutaneous candidosis, hyperplastic candidosis, oropharyngeal candidosis, and rare suppurative candidosis. The prognosis is usually favorable, but treatment failure or recurrence is common due to either incorrect diagnosis, missing other pathology, inability to address underlying risk factors, or inaccurate prescription of antifungal agents. In immunocompromised patients, oropharyngeal candidosis can spread to the bloodstream or upper gastrointestinal tract, leading to potentially lethal systemic candidosis. This review therefore describes oral candidosis with regard to its pathophysiology and best practice for diagnosis, practical classification, and successful management.
2. Candida Species
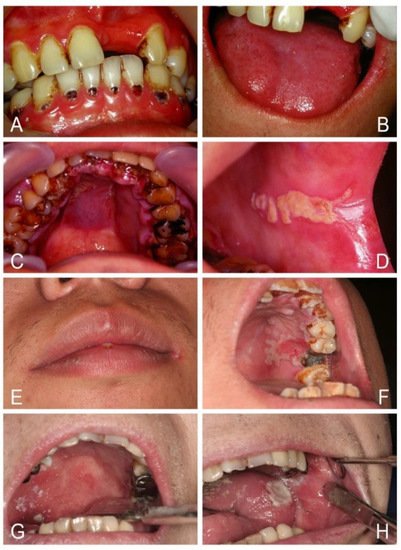
3. Conclusions
- (1)
-
Maintenance of good oral and denture hygiene is crucial. It is important to remove dentures overnight, use denture cleanser, or make a new denture if an ill-fitting denture with stomatitis exists.
- (2)
-
Rinsing the mouth after use of an inhaled steroid is helpful to prevent oral candidosis.
- (3)
-
Glucose promotes yeast growth, and a high-carbohydrate diet enhances its adherence to oral epithelial cells. Limiting their consumption is helpful in the control of oral Candida colonization and infection.
- (4)
-
Removal of heavy candidal plaques or biofilm from oral lesions by mechanical means can improve antifungal action and speed healing.
- (5)
-
Nystatin tablets are significantly superior to nystatin oral suspension in treating oral candidosis. Swallowing nystatin tablets rather than sucking or dissolving them in the mouth is ineffective to treat oral candidosis.
- (6)
-
The duration of antifungal treatment should be sufficient or prolonged for at least four weeks to achieve a more permanent mycological cure.
- (7)
-
Early fluconazole monotherapy or fluconazole combined with nystatin is helpful to treat oropharyngeal candidosis, suppurative candodosis, or Candida-related chronic oral ulcers.
- (8)
-
Attempts to increase CD4 count in patients with HIV/AIDS or thymoma are helpful to treat oral candidosis. HAART can reduce recurrent oropharyngeal candidosis.
- (9)
-
Underlying predisposing factors should be identified and treated simultaneously as well as monitored regularly.
- (10)
-
The final eradication of oral candidosis is by host defense system.
This entry is adapted from the peer-reviewed paper 10.3390/jof7070555
References
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15.
- Darwazeh, A.M.G.; Darwazeh, T.A. What makes oral candidiasis recurrent infection? A clinical view. J. Mycol. 2014, 2014, 1–5.
- Schsuer, F.; Hanschke, R. Taxonomy and ecology of the genus Candida. Mycoses 1999, 42, 12–21.
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459.
- Samaranayake, L.P.; MacFarlane, T.W. (Eds.) Oral Candidosis; Butterworth: London, UK, 1990; pp. 1–259.
- Ruhnke, M. Epidemiology of Candida albicans infections and role of non-Candida albicans yeasts. Curr. Drug Targets 2006, 7, 495–504.
- Supriya, H.; Harishchandra, R.; Suhasini, P.D.; Rajalekshmi, V. Pathogenic mechanism of Candida albicans in oral mucosa—A review. Int. J. Health Sci. Res. 2016, 1, 489–497.
- Rao, P.N.; Rao, K.N. Study of the normal conjunctival flora (bacterial and fungal) and its relations to external ocular infections. Indian I. Ophthalmol. 1972, 20, 164–170.
- Sthapit, P.R.; Tuladhar, N.R. Conjunctival flora of normal human eye. JSM Ophthalmol. 2014, 2, 1021.
- Alrayyes, S.F.; Alruwaili, H.M.; Taher, I.A.; Elrahawy, K.M.; Almaeen, A.H.; Ashekhi, A.O.; Alam, M.K. Oral candidal carriage and associated risk indicators among adults in Sakaka, Saudi Arabia. BMC Oral Health 2019, 22, 86.
- Zomorodian, K.; Kavoosi, F.; Pishdad, G.R.; Mehriar, P.; Ebrahimi, A.; Bandegani, A.; Pakshir, K. Prevalence of oral Candida colonization in patients with diabetes mellitus. J. Mycol. Med. 2016, 26, 103–110.
- Vidya, K.M.; Rao, U.K.; Nittayananta, W.; Liu, H.; Owotade, F.J. Oral mycoses and other oppontunistic infectionsin HIV: Therapy and emerging problems-a workshop report. Oral Dis. 2016, 22, 158–165.
- Schelenz, S.; Abdallah, S.; Gray, G.; Stubbings, H.; Gow, I.; Baker, P. Epidemiology of oral yeast colonization and infection in patients with hematological malignancies, head neck and solid tumors. J. Oral Pathol. Med. 2011, 40, 83–89.
- Rennie, J.S.; MacDonald, D.G.; Dagg, J.H. Iron and the oral epithelium: A review. J. R. Soc. Med. 1984, 77, 602–607.
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10.
- Kaur, R.; Dhakad, M.S.; Goyal, R.; Kumar, R. Emergence of non-albicans Candida species and antifungal resistance in intensive care unit patients. Asian Pac. J. Trop. Biomed. 2016, 6, 455–460.
- Quindós, G. Epidemiology of candidemia and invasive candidiasis. A changing face. Rev. Iberoam Micol. 2014, 31, 42–48.
- Patil, S.; Majumdar, B.; Sarode, S.C.; Sarode, G.S.; Awan, K.H. Oropharyngeal candidosis in HIV-infected patients—An update. Front. Microbiol. 2018, 9, 980.
- Thompson, G.R., III; Patel, P.K.; Kirkpatrick, W.R.; Westbrook, S.D.; Berg, D.; Erlandsen, J.; Redding, S.W.; Patterson, T.F. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 488–495.
- Yang, Y.L.; Lo, H.J. Mechanisms of antifungal agent resistance. J. Microbiol. Immunol. Infect. 2001, 34, 79–86.
- Mishra, N.N.; Prasad, T.; Sharma, N.; Payasi, A.; Prasad, R.; Gupta, D.K.; Singh, R. Pathogenicity and drug resistance in Candida albicans and other yeast species. Acta Microbiol. Immunol. Hung. 2007, 54, 201–235.
- Tobgi, R.S.; Samaranayake, L.P.; MacFariane, T.W. In vitro susceptibility of Candida species to lysozyme. Oral Microbiol. Immunol. 1988, 3, 36–39.
- Sawasdipuksa, N.; Lei, Z.; Sumner, L.W.; Niyomploy, P.; Sangvanich, P. A lysozyme with antifungal activity from Pithecellobium dulce seeds. Food Technol. Biotechnol. 2011, 49, 489–494.
- Galvez-Iriqui, A.C.; Plascencia-Jatomea, M. Lysozymes: Characteristics, mechanism of action and technological applications on the control of pathogenic microrganisms. Mex. J. Phytopathol. 2020, 38, 360–383.
- Redding, S.W.; Dahiya, C.; Kirkpatrick, W.R.; Coco, B.J.; Patterson, T.F.; Fothergill, A.W.; Rinaldi, M.G.; Thomas, C.R., Jr. Candida glabrata is an emerging cause of oropharyngeal candidiasis in patients receiving radiation for head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radio. Endod. 2004, 97, 47–52.
- Van Wyk, C.; Steenkamp, C. Host factors affecting oral candidiasis. South. Afr. J. Epidemiol. Infect. 2011, 26, 18–21.
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanism. Virulence 2013, 4, 119–128.
