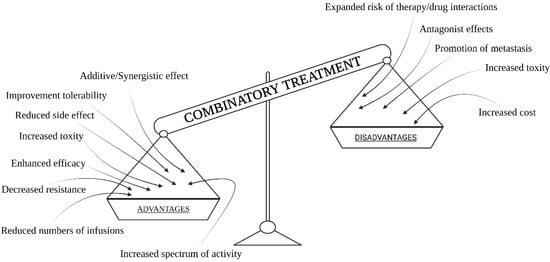
In the context of the use of combination therapies in cancer treatment, there are some very interesting results obtained by the use of chemotherapy, radiotherapy, hyperthermia, photodynamic therapy or surgery, and immunotherapy in various combinations. Currently, a combination treatment of chemotherapy, radiotherapy, and often surgery is used commonly in cancer treatment. With this approach, we can effectively stop cancer development and obtain the best possible results based on current knowledge (but one of three cancer cases still lead to patient death). There is no doubt that combination treatment provides very good results, as opposed to using only one method due to the comprehensiveness of the multi-module treatment. Based on many simulations with the use of computer models, cancer treatment strategies were optimized using a combination of possible treatments [
9,
10,
11]. The crucial elements for optimization are drugs and their doses, as well as the dose of light and radiation, in the context of cancer type and the degree of its aggressiveness. Fractionation of the radiation dose also proved to be more crucial, giving better results [
12,
13].
In addition, cutting out cancerous tissue, with a margin of healthy tissue, combined with chemotherapy cycles, provides the best results and reduces tumor metastasis. It also allows for targeted treatment and effectiveness increase due to a long-term and selective action. Taking into account the occurrence of cell cycles and cell resistance at various stages of the cell cycle for selected methods, it offers more significant results. Scientific research has also shown that the cells present in the phase that is the most resistant to radiation, i.e., those that are in the S phase (replication phase), are also very sensitive to hyperthermia [
14]. Therefore, it was concluded that hyperthermia allows sensitization of cells to X-rays in the further treatment phase [
15]. When considering combination therapy, one may come across concepts, such as thermochemotherapy, thermoradiotherapy, and thermochemoradiotherapy. Considering thermoradiotherapy, the general mechanism of action is primarily the possibility of destroying cancer cells that are insensitive to radiation. It also inhibits the process of repairing damage to DNA under the influence of a dose of ionizing radiation. Due to this approach, combination therapy in cancer treatment offers more effective therapeutic results. In turn, analyzing the introduced concept of thermochemotherapy in the treatment of cancer, one may observe an increase in sensitivity and reduction in cancer cell resistance to chemotherapy. With this approach, it is possible to increase the accumulation of chemotherapeutics in the cancer location [
15,
16]. All of this contributes toward increasing the effectiveness of this complementary method, which, in this context, has been linked to phenomena, such as hyperthermia. Undoubtedly, new classes of chemotherapeutics, as well as various mechanisms of action of drugs, such as the use of drugs that are not dependent on the rate of proliferation, or those that depend on the phase of the cell cycle, and attempts to combine drugs with non-overlapping toxicity, or a use of a combination of drugs that have different mechanisms of action [
17], as well as the way of adding drugs, for example using targeted liposomes, significantly improve the effects of treatment and show us the importance of using combination therapy. On the other hand, the use of thermochemoradiotherapy shows that multi-module treatment brings the most beneficial and the best results [
18]. Researchers have studied the interaction between hyperthermia and radiation therapy, as well as chemotherapy using chemotherapeutic agents, such as cisplatin. Scientific research has shown that a synergistic effect can be obtained, but only when this three-modular therapy is used on cell lines that are not resistant to cisplatin [
19,
20]. Considering photodynamic therapy, this combination of therapy with chemotherapy gives quite good results due to direct destruction of cancer cells and strong influence on blood vessels in the tumor surrounding. It was shown that different PDT protocols can significantly damage the vasculature or temporally increase blood flow and tissue oxygenation in the treated area [
21]. However, as a result of photodynamic therapy, when not all of the cells are destroyed, the use of chemotherapy in the second wave allows killing the remaining cancer cells that have survived PDT. Additionally, it was shown that PDT can have strong influence on the immune system [
22]. With this approach, combining methods can stop the cancer or lead to a complete cure.