Glucose is a major macronutrient and a vital homeostatic factor in the regulation of energy metabolism maintained in a narrow range of 4.4 to 6.1 mmol/L or about 1.0 g/L in the blood of healthy humans as measured in the fasting state. However, glucose per se is not the predominant component of mixed food, and its main source in the diet is poly- and oligosaccharides, which undergo enzymatic hydrolysis to monomers in the small intestine during luminal and membrane digestion. Depending on the food composition, the site of the gastrointestinal tract (GIT) and time of the day, the postprandial glucose concentrations in the GIT lumen can vary in a large range and can be several times higher than in the blood.
- glucose
- small intestine
- insulin resistance
- gut-brain axis
- appetite
- obesity metabolic syndrome
- type 2 diabetes mellitus
1. Introduction
In rats fed with 65% glucose diet, the highest concentration of free glucose was found in the stomach (average about 1000 mmol/L during the day), while in the lumen of the proximal and distal small intestine it was about 50 and 1.0 mmol/L, respectively. In humans, concentration of gut luminal glucose about 48 mmol/L, was found in the upper intestine samples taken 2 h after eating a meal [1]. This shows that the gut provides both a barrier by limiting glucose passage and a regulatory mechanism for maintaining blood glucose levels. Thus, glucose absorption together with glucose ingestion and metabolism are all the interconnected processes determining the blood glucose levels and its availability to organs and tissues.
Disturbed regulation of the blood glucose levels leading to hyperglycemia, is a central problem of the pathophysiology of metabolic diseases such as obesity, metabolic syndrome and type 2 diabetes (T2D). Hyperphagia is a characteristic feature of these diseases suggesting a possible link to the regulation of blood glucose. In fact, the role of glucose as a satiety factor in the short-term regulation of appetite is well-known and was the basis for glucostatic theory of appetite [2]. Thus, molecular and cellular mechanisms underlying the dynamics of absorption of glucose (free or formed during the hydrolysis of complex carbohydrates) in the small intestine may impact on fluctuation of plasma glucose levels relevant to the regulation of appetite. However, it is not clear if targeting glucose absorption mechanism can be beneficial for treatment of hyperphagia in metabolic disease.
In recent years, various pathways of glucose transfer across the epithelium of the small intestine were studied concerning their contribution to the resultant absorption of glucose. Presently, most researchers agree that at relatively low glucose concentrations in the intestinal lumen in vivo (less than 30 mM), glucose (as well as galactose), is transferred across the apical membrane of enterocytes by active transport mediated by the transporter SGLT1, while its exit into the blood flow is carried out by the facilitated diffusion mediated by the transporter GLUT2, localized in the basolateral membrane [3][4][5].
At high luminal concentrations of glucose (more than 30 mM), the active transport of glucose becomes saturated and the other mechanisms might be involved in the absorption of glucose in the small intestine. It was hypothesized that one of these mechanisms may involve paracellular transfer through the tight junctions using a flow of absorbed water (‘solvent drag’ mechanism) [6]. Later, another hypothesis has been put forward that at high carbohydrate loads, the GLUT2 transporters can be quickly incorporated into the brush border membrane of enterocytes and participate in facilitated diffusion of glucose across this membrane [3]. The exact contribution of each of these mechanisms to the total absorption of glucose at its high concentrations in the intestinal lumen under normal conditions, as well as in metabolic disorders, needs further clarification.
Several studies have shown that glucose absorption as well as the expression and activity of the SGLT1 and GLUT2 glucose transporters in enterocytes are increased in diabetes [5], suggesting that it may contribute to hyperglycemia. Given the predominant role of SGLT1 transporters in the absorption of glucose in the small intestine, it is reasonable to consider these transporters as targets for the treatment of T2D. Indeed, recent use of new SGLT1 inhibitors was shown to reduce blood glucose levels and improve metabolic parameters in T2D patients without serious gastrointestinal side effects [7]. Nevertheless, despite a successful approach for lowering blood glucose levels, it is not clear if the inhibition of small intestinal glucose absorption may affect appetite and if can be useful for the treatment of hyperphagia.
The physiological studies showed that the expression and activity of the SGLT1 and GLUT2 transporters in small intestinal enterocytes undergo both short- and long-term regulation by dietary carbohydrates as well as by regulatory factors, including peptide hormones involved in the regulation of appetite such as leptin, glucagon-like peptide-1 (GLP-1) etc. [8]. However, the reported effects of such factors are often ambiguous, and in the case of GLUT2, they are even rare.
2. Glucose Absorption and Appetite Regulation
Glucose absorption can be considered an integral part of the homeostatic system maintaining blood glucose levels, where it may provide the negative feedback to the brain control of food intake. Nevertheless, it should be noted that such homeostatic system can be overrun by the hedonic regulation of appetite, whereas highly pleasurable glucose intake my trigger new intakes via activation of the brain dopamine [9]. The relative changes or fluctuations of blood glucose levels as determined by simultaneous processes of glucose supply and utilization served as the basis for the “glucostatic” theory of appetite proposed by Jean Mayer in the 1950s [2]. In this section, we will discuss the possible relevance of small intestinal glucose absorption to the control of appetite and feeding behavior i.e., the physiological functions necessary for nutrient intake and glucose metabolism in both healthy and disease conditions ( Figure 2 ).
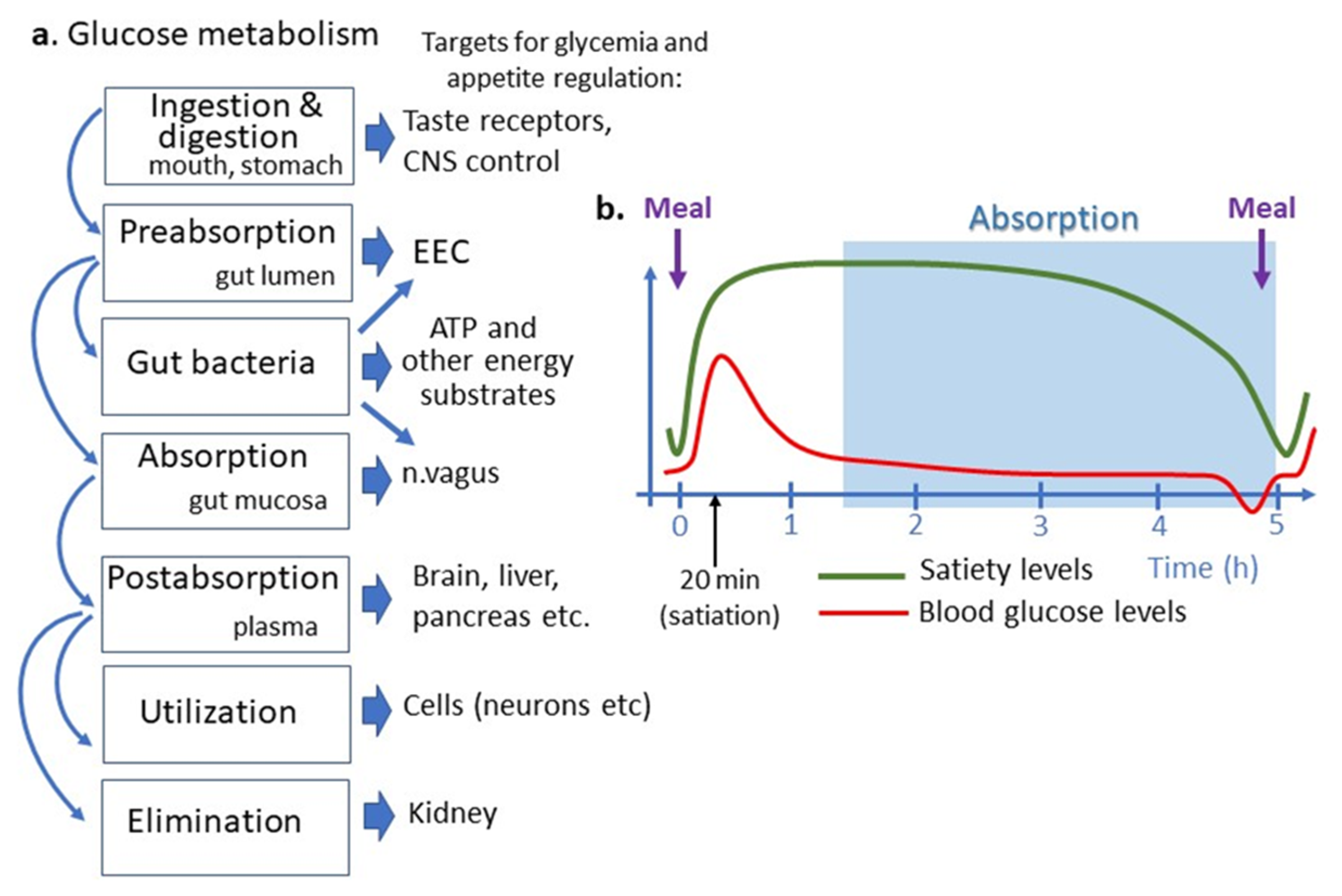 Figure 2. Place of intestinal glucose absorption in glucose metabolisms and regulation of appetite. (a) Different steps of glucose metabolism correspond to different targets for appetite regulation. (b) Temporal relation between appetite cycles shown as satiety levels, blood glucose levels and an approximate period of intestinal nutrient absorption. Note that the small intestinal glucose absorption occurs after gastric emptying and appearance of satiation i.e., later than postprandial glucose peak, which is due to reflectory hepatic glucose production. Intestinal glucose absorption continues during a short preprandial fall in blood glucose and appearance of hunger feeling. ATP, adenosine triphosphate, CNS, central nervous system, EEC, enteroendocrine cells.
Figure 2. Place of intestinal glucose absorption in glucose metabolisms and regulation of appetite. (a) Different steps of glucose metabolism correspond to different targets for appetite regulation. (b) Temporal relation between appetite cycles shown as satiety levels, blood glucose levels and an approximate period of intestinal nutrient absorption. Note that the small intestinal glucose absorption occurs after gastric emptying and appearance of satiation i.e., later than postprandial glucose peak, which is due to reflectory hepatic glucose production. Intestinal glucose absorption continues during a short preprandial fall in blood glucose and appearance of hunger feeling. ATP, adenosine triphosphate, CNS, central nervous system, EEC, enteroendocrine cells.
Glucose may also play the role of a short-term satiety signal which plasma levels rise after each meal and returns to the preprandial levels after 1–2 h [11] ( Figure 2 ). Increased plasma levels of glucose and several gut peptides activate neuronal satiety pathways and contribute to satiety mechanisms by inhibiting gastric emptying [12]. Some data, however, suggest that postprandial levels of plasma insulin rather than of glucose are associated with satiety [13]. Importantly, the different dynamics of postprandial glucose peak and glucose absorption do not support the latter to play a role in satiety signaling. In fact, in the postprandial period, glucose absorptions in the intestine begin after the gastric emptying which occurs 1–2 h after a meal, i.e., when the organism has been in the satiety state ( Figure 2 ). The meal-induced peak of plasma glucose appears, hence, as an absorption-independent result of a neuronal reflex mechanism to nutrient ingestion involving mainly acute activation of hepatic glucose production [14]. The physiological significance of such mechanisms may be the anticipation of carbohydrate absorption extracted from nutrients and their storage as glycogen in the liver [15].
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/nu13072474
References
- Ferraris, R.P.; Yasharpour, S.; Lloyd, K.C.; Mirzayan, R.; Diamond, J.M. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol. Liver Physiol. 1990, 259, G822–G837.
- Mayer, J. Glucostatic mechanism of regulation of food intake. N. Engl. J. Med. 1953, 249, 13–16.
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of GLUT2. Annu. Rev. Nutr. 2008, 28, 35–54.
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794.
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020, 472, 1207–1248.
- Pappenheimer, J.R. Paracellular intestinal absorption of glucose, creatinine, and mannitol in normal animals: Relation to body size. Am. J. Physiol. Liver Physiol. 1990, 259, G290–G299.
- Cefalo, C.M.A.; Cinti, F.; Moffa, S.; Impronta, F.; Sorice, G.P.; Mezza, T.; Pontecorvi, A.; Giaccari, A. Sotagliflozin, the first dual SGLT inhibitor: Current outlook and perspectives. Cardiovasc. Diabetol. 2019, 18, 1–14.
- Chan, L.K.Y.; Leung, P.S. Multifaceted interplay among mediators and regulators of intestinal glucose absorption: Potential impacts on diabetes research and treatment. Am. J. Physiol. Metab. 2015, 309, E887–E899.
- Fernandes, A.B.; Alves da Silva, J.; Almeida, J.; Cui, G.; Gerfen, C.R.; Costa, R.M.; Oliveira-Maia, A.J. Postingestive modulation of food seeking depends on vagus-mediated dopamine neuron activity. Neuron 2020, 106, 778–788.e6.
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671.
- Daly, M.E.; Vale, C.; Walker, M.; Littlefield, A.; Alberti, K.G.; Mathers, J.C. Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high-starch diet. Am. J. Clin. Nutr. 1998, 67, 1186–1196.
- Rayner, C.K.; Samsom, M.; Jones, K.L.; Horowitz, M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001, 24, 371–381.
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25.
- Seydoux, J.; Brunsmann, M.J.; Jeanrenaud, B.; Girardier, L. Alpha-sympathetic control of glucose output of mouse liver perfused in situ. Am. J. Physiol. Endocrinol. Metab. 1979, 236, E323.
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: An Integrative approach. Nutrients 2021, 13, 159.
- Déchelotte, P.; Breton, J.; Trotin-Picolo, C.; Grube, B.; Erlenbeck, C.; Bothe, G.; Fetissov, S.O.; Lambert, G. The probiotic strain H. alvei HA4597® improves weight loss in overweight subjects under moderate hypocaloric diet: A proof-of-concept, multicenter randomized, double-blind placebo-controlled study. Nutrients 2021, 13, 1902.
