Causal agents of schistosomiasis are dioecious, digenean schistosomes affecting mankind in 76 countries. Natural and anthropogenic changes impact on breaking species isolation barriers favoring introgressive hybridization, i.e., allelic exchange among gene pools of sympatric, interbreeding species leading to instant large genetic diversity. Phylogenetic distance matters, thus the less species differ phylogenetically the more likely they hybridize.
- hybridization
- introgression
- immunization
- Africa
1. Schistosomiasis and Parasite Hybridization
Schistosomiasis affects mankind in 76 countries causing an estimated annual mortality rate of 280,000, 779 million people at risk of infection, 250 million people with active infections, and 440 million people with residual morbidity [1][2][3][4]. The disease burden is largest throughout sub-Saharan Africa accounting for >90% caused by the clinically most relevant human-pathogenic species Schistosoma mansoni (Sm) and S. haematobium (Sh) [1][2][3][4]. Though schistosomiasis is likely underrecognized among the veterinary sector, inclusive of livestock and wildlife animals, it is assumed that in particular ruminants, rodents and non-human primates are carriers of zoonotic species [5].
The causal agents of this neglected tropical disease are dioecious digenean schistosomes within the Platyhelminthes; species by clade of the Schistosoma genus including their geographical distribution, and intermediate and definitive hosts are illustrated in Figure 1 [5][6][7][8].
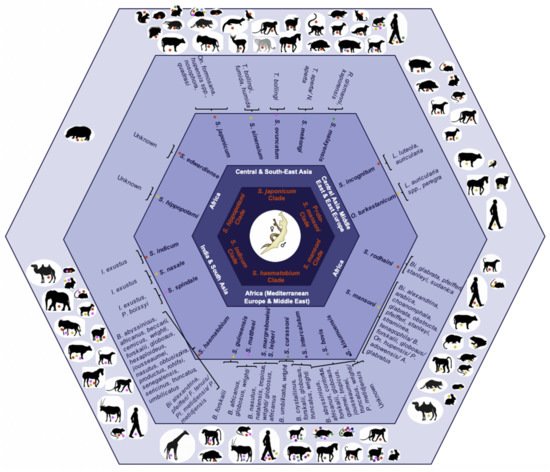
2. Schistosome Hybrids across Africa
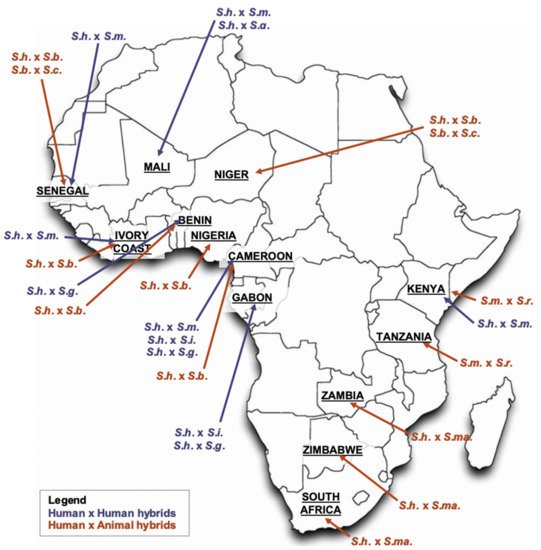
2.1. S. haematobium/S. guineensis Hybrids
Interactions between Sh and Sg are reported from South-West Cameroon; of note is the re-description of S. intercalatum (Si) from Lower Guinea, e.g., Cameroon, Gabon, Equatorial Guinea and São Tomé, as Sg [22][23][28][29][30]. Ecological changes such as forest clearance and agricultural development evolving in the 1960s besides population movement favored the presence of snail hosts, e.g., Bulinus wrighti, B. forskalii and B. truncatus, and species sympatry and interplay; this materialized the complete replacement of Sg by Sh within 25–30 years through introgressive hybridization and competitive extinction abetted by behavioral, reproductive and genetic advantages [31][32][33]. Experiments confirmed greater competitiveness and pairing abilities of ShxSg crosses over parental species, and Sh being dominant over Sg, which supports observations made in Cameroon and elsewhere, e.g., Kinshasa, Democratic Republic of Congo, and Dogon Country, Mali [34][35][36][37]. Retrospective experiments among schistosomes of laboratory maintained urinary ova of Cameroonian children detected hybridization between Sh and Sg among 100%, 33% and 5% isolates from Kumba, Loum (1990), and Loum (1999 and 2000), respectively; the remainder had pure Sh, but no Sg despite established B. forskalii hosts [28]. Findings were confirmed by four-banded single-stranded conformational polymorphism (SSCP) profiles of second nuclear ribosomal internal transcribed spacer (ITS2) fragments. Sequencing isolates from Loum revealed unequivocal intermediate SNPs at the positions 25, 80 and 130 within ITS2 fragments compared to parental strains, i.e., guanine-cytosine-guanine for Sh, and adenine-thymine-adenine for Sg, clearly demonstrating ShxSg recombinants with dominance, thus inheritance, of Sh over Sg [23].
2.2. S. haematobium/S. bovis Hybrids
2.3. S. haematobium/S. mansoni Hybrids
2.4. S. mansoni/S. rodhaini Hybrids
3. Schistosomal Hybridization—Interference on Leading Vaccine Candidates
Extensive small-scale gene amplifications and alterations are contributing to a steady schistosomal genomic evolution [27]. SNPs emerging through transversional/transitional or synonymous/non-synonymous nucleotide substitutions leading to e.g., alternative splicing or silent mutations are of high importance in particular among sequences serving as drug or vaccine targets; they may lead to structural and functional protein alterations impacting charge changes and residue conservation affecting drug binding sites or antibody recognition of antigenic epitopes [55][56][57]. Hosts react in the following general manners against schistosomal infections, i.e., development of age-dependent partial protective immunity to reinfection from repeated adult worm death, and initiation of immunopathogenic and/or immunoregulatory mechanisms against parasitic antigens released from eggs trapped in tissues [58][59][60].
Preliminary findings of the phase 2a trial confirmed rSm14+GLA-SE’s safety and strong, long-lasting immunogenicity, and reported 92% seroconversion following the second dose with cellular responses, memory cells and T-cell activation makers [61]. However, Sm14 isoforms demonstrate the potential of structural instability resulting in its aggregation and precipitation and, in turn, to uncertain immunoprotection and functional alterations [57][62].
rSh28GST+Alhydrogel/Bilhvax® vaccine candidate was tested in a phase 3 safety, efficacy, disease recurrence and immunogenicity trial among Sh infected schoolchildren from the ShxSb and ShxSm hybrid endemic SRB receiving two-dose PZQ pre-treatment, three-dose immunization, and a single-dose PZQ treatment prior to a final vaccine booster dose [63][64][65][66]. rSh28GST+Alhydrogel proved its safety, strong, long-lasting immunogenicity, and anti-worm fecundity with inhibited egg viability despite the fact that the primary endpoint of significant delay in schistosomiasis recurrence was not reached in the trial [63].
Of interest related to the ShxSm hybrid type could be the potential of the Smp80-GLA-SE/SchistoShield® vaccine candidate to confer robust, balanced Th1/Th2 cross-species prophylactic and therapeutic protection against Sh in addition to Sj as demonstrated in rodents and baboons [67][68]. This observation could be due to 95% and 84% amino acid homology among calpain of Sh and Sj with Sm, respectively. Likewise, an ongoing phase 1 dose-escalation, safety and immunogenicity Sm-TSP-2/Sm-TSP-2Al® trial coupled with a subsequent 2b Sm-TSP-2/Sm-TSP-2Al® trial among Sm and Sh exposed healthy male and female Ugandan adults receiving three-dose immunization could reveal promising cross-species protective effects depending on the level of inter- and/or intra-species polymorphism of the TSP-2 EC2 domain [69].
4. Conclusions
A vaccine as the current candidate in advanced to pre-clinical and clinical phases with cross-species protective potential is needed to complement preventive measures, and protect long-term against transmission, infection, and disease recurrence and sequelae. Despite the strong evidence of naturally occurring schistosomal hybrids of large functional and structural diversity, its potential impact on current vaccine candidates is yet unclear and requires further research. Vaccine targets exposed at the schistosome tegumental surface should not evolve rapidly leading to sequence heterogenicity in natural parasite populations, and rendering its use as a vaccine candidate ineffective, but rather highly conserved. Additionally, natural history studies seem of particular importance in areas with known endemicity for hybrid schistosomes that are targeted for clinical trials.This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9071465
References
- Nelwan, M.L. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr. Ther. Res. 2019, 91, 5–9.
- Webster, J.P.; Neves, M.I.; Webster, B.L.; Pennance, T.; Rabone, M.; Gouvras, A.; Allan, F.; Walker, M.; Rollinson, D. Parasite Population Genetic Contributions to the Schistosomiasis Consortium for Operational Research and Evaluation within Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2020, 103, 80–91.
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264.
- Molehin, A.J. Schistosomiasis vaccine development: Update on human clinical trials. J. Biomed. Sci. 2020, 27, 1–7.
- Leger, E.; Webster, J.P. Hybridizations within the Genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology 2017, 144, 65–80.
- Lawton, S.P.; Hirai, H.; Ironside, J.E.; Johnston, D.A.; Rollinson, D. Genomes and geography: Genomic insights into the evolution and phylogeography of the genus Schistosoma. Parasites Vectors 2011, 4, 131.
- Webster, B.; Southgate, V.; Littlewood, D. A revision of the interrelationships of Schistosoma including the recently described Schistosoma guineensis. Int. J. Parasitol. 2006, 36, 947–955.
- London NHM. Host-Parasite Database. Available online: (accessed on 2 June 2021).
- Pearce, E.J.; Macdonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511.
- Vale, N.; Gouveia, M.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; da Costa, J.M.C. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16.
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Lafferty, K.D.; Kuris, A.M.; Hsieh, M.H.; De Leo, G.A. To Reduce the Global Burden of Human Schistosomiasis, Use ‘Old Fashioned’ Snail Control. Trends Parasitol. 2018, 34, 23–40.
- Merrifield, M.; Hotez, P.J.; Beaumier, C.M.; Gillespie, P.; Strych, U.; Hayward, T.; Bottazzi, M.E. Advancing a vaccine to prevent human schistosomiasis. Vaccine 2016, 34, 2988–2991.
- Dumont, M.; Moné, H.; Mouahid, G.; Idris, M.A.; Shaban, M.; Boissier, J. Influence of pattern of exposure, parasite genetic diversity and sex on the degree of protection against reinfection with Schistosoma mansoni. Parasitol. Res. 2007, 101, 247–252.
- King, K.; Stelkens, R.B.; Webster, J.P.; Smith, D.F.; Brockhurst, M.A. Hybridization in Parasites: Consequences for Adaptive Evolution, Pathogenesis, and Public Health in a Changing World. PLoS Pathog. 2015, 11, e1005098.
- Léger, E.; Garba, A.; Hamidou, A.A.; Webster, B.; Pennance, T.; Rollinson, D.; Webster, J.P. Introgressed Animal Schistosomes Schistosoma curassoni and S. bovis Naturally Infecting Humans. Emerg. Infect. Dis. 2016, 22, 2212–2214.
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571.
- Webster, B.; Diaw, O.T.; Seye, M.M.; Webster, J.P.; Rollinson, D. Introgressive Hybridization of Schistosoma haematobium Group Species in Senegal: Species Barrier Break Down between Ruminant and Human Schistosomes. PLoS Negl. Trop. Dis. 2013, 7, e2110.
- Crego-Vicente, B.; Fernández-Soto, P.; Febrer-Sendra, B.; Diego, J.G.-B.; Boissier, J.; Angora, E.; Oleaga, A.; Muro, A. Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium × Schistosoma bovis Hybrids. J. Clin. Med. 2021, 10, 1308.
- Rey, O.; Toulza, E.; Chaparro, C.; Allienne, J.-F.; Kincaid-Smith, J.; Mathieu-Begné, E.; Allan, F.; Rollinson, D.; Webster, B.L.; Boissier, J. Diverging patterns of introgression from Schistosoma bovis across S. haematobium African lineages. PLoS Pathog. 2021, 17, e1009313.
- Steinauer, M.L.; Blouin, M.S.; Criscione, C.D. Applying evolutionary genetics to schistosome epidemiology. Infect. Genet. Evol. 2010, 10, 433–443.
- Steinauer, M.L.; Hanelt, B.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Kinuthia, J.M.; Mutuku, M.W.; Mungai, B.N.; Wilson, W.D.; Mkoji, G.M.; et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol. Ecol. 2008, 17, 5062–5074.
- Moné, H.; Minguez, S.; Ibikounlé, M.; Allienne, J.F.; Massougbodji, A.; Mouahid, G. Natural interactions between S. haematobium and S. guineensis in the Republic of Benin. Sci. World J. 2012, 2012, 793420.
- Webster, B.L.; Tchuenté, L.A.T.; Southgate, V.R. A single-strand conformation polymorphism (SSCP) approach for investigating genetic interactions of Schistosoma haematobium and Schistosoma guineensis in Loum, Cameroon. Parasitol. Res. 2007, 100, 739–745.
- Rollinson, D. Biochemical genetics in the study of schistosomes and their intermediate hosts. Parassitologia 1985, 27, 123–139.
- Wright, C.; Ross, G. Hybrids between Schistosoma haematobium and S. mattheei and their identification by isoelectric focusing of enzymes. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 326–332.
- Catalano, S.; Sène, M.; Diouf, N.D.; Fall, C.B.; Borlase, A.; Léger, E.; Bâ, K.; Webster, J.P. Rodents as natural hosts of zoonotic schistosoma species and hybrids: An epidemiological and evolutionary perspective from West Africa. J. Infect. Dis. 2018, 218, 429–433.
- Wang, S.; Zhu, X.-Q.; Cai, X. Gene Duplication Analysis Reveals No Ancient Whole Genome Duplication but Extensive Small-Scale Duplications during Genome Evolution and Adaptation of Schistosoma mansoni. Front. Cell. Infect. Microbiol. 2017, 7, 412.
- Webster, B.; Tchuenté, L.T.; Jourdane, J.; Southgate, V. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005, 79, 193–197.
- Wright, C.A.; Southgate, V.R.; Van Wijk, H.B.; Moore, P.J. Letter: Hybrids between Schistosoma haematobium and S. intercalatum in Cameroon. Trans. R. Soc. Trop. Med. Hyg. 1974, 68, 413–414.
- Webster, B.; Southgate, V.; Tchuenté, L.-A.T. Isoenzyme analysis of Schistosoma haematobium, S. intercalatum and their hybrids and occurrences of natural hybridization in Cameroon. J. Helminthol. 2003, 77, 269–274.
- Southgate, V.R.; van Wijk, H.B.; Wright, C.A. Schistosomiasis at Loum, Cameroun; Schistosoma haematobium, S. intercalatum and their natural hybrid. Parasitol. Res. 1976, 49, 145–159.
- Tchuem Tchuenté, L.A.; Southgate, V.R.; Njiokou, F.; Njiné, T.; Kouemeni, L.E.; Jourdane, J. The evolution of schistosomiasis at Loum, Cameroon: Replacement of Schistosoma intercalatum by S. haematobium through introgressive hybridization. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 664–665.
- Southgate, V.R. On factors possibly restricting the distribution of Schistosoma intercalatum Fisher, 1934. Parasitol. Res. 1978, 56, 183–193.
- Cosgrove, C.L.; Southgate, V.R. Competitive mating interactions between Schistosoma haematobium and S. intercalatum (Lower Guinea strain). Parasitol. Res. 2003, 89, 238–241.
- Webster, B.L.; Southgate, V.R. Mating interactions of Schistosoma haematobium and S. intercalatum with their hybrid offspring. Parasitology 2003, 126, 327–338.
- Tchuem Tchuenté, L.A.; Southgate, V.R.; Vercruysse, J.; Kaukas, A.; Kane, R.; Mulumba, M.P.; Pagès, J.R.; Jourdane, J. Epidemiological and genetic observations on human schistosomiasis in Kinshasa, Zaire. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 263–269.
- Vercruysse, J.; Southgate, V.R.; Rollinson, D.; De Clercq, D.; Sacko, M.; De Bont, J.; Mungomba, L.M. Studies on transmission and schistosome interactions in Senegal, Mali and Zambia. Trop. Geogr. Med. 1994, 46, 220–226.
- Picquet, M.; Ernould, J.C.; Vercruysse, J.; Southgate, V.R.; Mbaye, A.; Sambou, B.; Niang, M.; Rollinson, D. Royal Society of Tropical Medicine and Hygiene Meeting at Manson House, London, 18 May 1995. The epidemiology of human schistosomiasis in the Senegal river basin. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 340–346.
- Southgate, V. Schistosomiasis in the Senegal River Basin: Before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J. Helminthol. 1997, 71, 125–132.
- Van den Broeck, F.; Maes, G.E.; Larmuseau, M.H.D.; Rollinson, D.; Sy, I.; Faye, D.; Volckaert, F.A.M.; Polman, K.; Huyse, T. Reconstructing colonization dynamics of the human parasite Schistosoma mansoni following anthropogenic environmental changes in Northwest Senegal. PLoS Negl. Trop. Dis. 2015, 9, e0003998.
- Boon, N.A.; Mbow, M.; Paredis, L.; Moris, P.; Sy, I.; Maes, T.; Webster, B.; Sacko, M.; Volckaert, F.A.; Polman, K.; et al. No barrier breakdown between human and cattle schistosome species in the Senegal River Basin in the face of hybridisation. Int. J. Parasitol. 2019, 49, 1039–1048.
- Platt, R.; McDew-White, M.; Le Clec’H, W.; Chevalier, F.; Allan, F.; Emery, A.M.; Garba, A.; Hamidou, A.A.; Ame, S.M.; Webster, J.P.; et al. Ancient Hybridization and Adaptive Introgression of an Invadolysin Gene in Schistosome Parasites. Mol. Biol. Evol. 2019, 36, 2127–2142.
- Boon, N.A.; Van Den Broeck, F.; Faye, D.; Volckaert, F.A.; Mboup, S.; Polman, K.; Huyse, T. Barcoding hybrids: Heterogeneous distribution of Schistosoma haematobium × Schistosoma bovis hybrids across the Senegal River Basin. Parasitology 2018, 145, 634–645.
- Boon, N.A.M.; Fannes, W.; Rombouts, S.; Polman, K.; Volckaert, F.A.M.; Huyse, T. Detecting hybridization in African schistosome species: Does egg morphology complement molecular species identification? Parasitology 2017, 144, 954–964.
- Boissier, J.; Grech-Angelini, S.; Webster, B.; Allienne, J.-F.; Huyse, T.; Mas-Coma, S.; Toulza, E.; Barré-Cardi, H.; Rollinson, D.; Kincaid-Smith, J.; et al. Outbreak of urogenital schistosomiasis in Corsica (France): An epidemiological case study. Lancet Infect. Dis. 2016, 16, 971–979.
- Angora, E.K.; Allienne, J.F.; Rey, O.; Menan, H.; Touré, A.O.; Coulibaly, J.T.; Raso, G.; Yavo, W.; N’Goran, E.K.; Utzinger, J.; et al. High prevalence of Schistosoma haematobium × Schistosoma bovis hybrids in schoolchildren in Cote d’Ivoire. Parasitology 2019, 147, 287–294.
- Webster, B.L.; Alharbi, M.H.; Kayuni, S.; Makaula, P.; Halstead, F.; Christiansen, R.; Juziwelo, L.; Stanton, M.C.; LaCourse, J.; Rollinson, D.; et al. Schistosome Interactions within the Schistosoma haematobium Group, Malawi. Emerg. Infect. Dis. 2019, 25, 1245–1247.
- Pitchford, R. Observations on a possible hybrid between the two schistosomes S. haematobium and S. mattheei. Trans. R. Soc. Trop. Med. Hyg. 1961, 55, 44–51.
- Van Wyk, J.A. The importance of animals in human schistosomiasis in South Africa. S. Afr. Med. J. 1983, 63, 201–203.
- Kruger, F.J.; Schutte, C.H.; Visser, P.S.; Evans, A.C. Phenotypic differences in Schistosoma mattheei ova from populations sympatric and allopatric to S. haematobium. Onderstepoort J. Vet. Res. 1986, 53, 103–107.
- Kruger, F.J.; Hamilton-Attwell, V.L. Scanning electron microscope studies of miracidia suggest introgressive hybridization between Schistosoma haematobium and S. haematobium × S. mattheei in the Eastern Transvaal. J. Helminthol. 1988, 62, 141–147.
- Huyse, T.; Broeck, F.V.D.; Hellemans, B.; Volckaert, F.; Polman, K. Hybridisation between the two major African schistosome species of humans. Int. J. Parasitol. 2013, 43, 687–689.
- Norton, A.J.; Webster, J.P.; Kane, R.A.; Rollinson, D. Inter-specific parasite competition: Mixed infections of Schistosoma mansoni and S. rodhaini in the definitive host. Parasitology 2008, 135, 473–484.
- Thèron, A. Hybrids between Schistosoma mansoni and S. rodhaini: Characterization by cercarial emergence rhythms. Parasitology 1989, 99, 225–228.
- Vilar, M.M.; Barrientos, F.; Almeida, M.; Thaumaturgo, N.; Simpson, A.; Garratt, R.; Tendler, M. An experimental bivalent peptide vaccine against schistosomiasis and fascioliasis. Vaccine 2003, 22, 137–144.
- Simões, M.; Bahia, D.; Zerlotini, A.; Torres, K.; Artiguenave, F.; Neshich, G.; Kuser, P.; Oliveira, G. Single nucleotide polymorphisms identification in expressed genes of Schistosoma mansoni. Mol. Biochem. Parasitol. 2007, 154, 134–140.
- Ramos, C.R.R.; Figueredo, R.C.R.; Pertinhez, T.A.; Vilar, M.M.; Nascimento, A.L.T.O.D.; Tendler, M.; Raw, I.; Spisni, A.; Ho, P.L. Gene Structure and M20T Polymorphism of the Schistosoma mansoni Sm14 Fatty Acid-binding Protein. J. Biol. Chem. 2003, 278, 12745–12751.
- Fukushige, M.; Mutapi, F.; Woolhouse, M.E. Population level changes in schistosome-specific antibody levels following chemotherapy. Parasite Immunol. 2019, 41, e12604.
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371.
- Panzner, U.; Excler, J.L.; Kim, J.H.; Marks, F.; Carter, D.; Siddiqui, A.A. Recent advances and methodological considerations on vaccine candidates for human schistosomiasis. Front. Trop. Dis. 2021. submitted.
- Tendler, M.; Almeida, M.S.; Vilar, M.M.; Pinto, P.M.; Limaverde-Sousa, G. Current Status of the Sm14/GLA-SE Schistosomiasis Vaccine: Overcoming Barriers and Paradigms towards the First Anti-Parasitic Human (itarian) Vaccine. Trop. Med. Infect. Dis. 2018, 3, 121.
- Ramos, C.R.R.; Spisni, A.; Oyama, S.; Sforça, M.; Ramos, H.R.; Vilar, M.M.; Alves, A.C.; Figueredo, R.C.R.; Tendler, M.; Zanchin, N.; et al. Stability improvement of the fatty acid binding protein Sm14 from S. mansoni by Cys replacement: Structural and functional characterization of a vaccine candidate. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 655–662.
- Riveau, G.; Schacht, A.-M.; Dompnier, J.-P.; Deplanque, D.; Seck, M.; Waucquier, N.; Senghor, S.; Delcroix-Genete, D.; Hermann, E.; Idris-Khodja, N.; et al. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: A phase 3 randomized, controlled trial in Senegalese children. PLOS Neglected Trop. Dis. 2018, 12, e0006968.
- Medicine/ClinicalTrials.gov USNLo. Efficacy and Safety Evaluation of the Therapeutic Vaccine Candidate Sh28GST in Association with Praziquantel (PZQ) for Prevention of Clinical and Parasitological Recurrences of S. haematobium Infection in Children [NCT00870649]. 2012. Available online: (accessed on 19 June 2021).
- Medicine/ClinicalTrials.gov USNLo. Safety and Immunogenicity Evaluation of the Vaccine Candidate Sm14 in Combination with the Adjuvant Glucopyranosyl Lipid A (GLA-SE) in Adults Living in Endemic Regions for S. Mansoni and S. haematobium in Senegal. A Comparative, Randomized, Open-Label Trial [NCT03041766]. 2016. Available online: (accessed on 19 June 2021).
- Medicine/ClinicalTrials.gov USNLo. Safety and Immunogenicity Evaluation of the Vaccine Candidate Sm14 against Schistosomiasis in Senegalese School Children Healthy or Infected with S. Mansoni and/or S. haematobium. A Comparative, Randomized, Controlled, Open-label Trial [NCT03799510]. 2018. Available online: (accessed on 19 June 2021).
- Molehin, A.J.; Sennoune, S.R.; Zhang, W.; Rojo, J.U.; Siddiqui, A.J.; Herrera, K.A.; Johnson, L.; Sudduth, J.; May, J.; Siddiqui, A.A. Cross-species prophylactic efficacy of Sm-p80-based vaccine and intracellular localization of Sm-p80/Sm-p80 ortholog proteins during development in Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Parasitol. Res. 2017, 116, 3175–3188.
- Karmakar, S.; Zhang, W.; Ahmad, G.; Torben, W.; Alam, M.U.; Le, L.; Damian, R.T.; Wolf, R.F.; White, G.L.; Carey, D.W.; et al. Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons. Vaccine 2014, 32, 1296–1303.
- Medicine/ClinicalTrials.gov USNLo. Sm-TSP-2 Schistosomiasis Vaccine in Healthy Ugandan Adults [NCT03910972]. 2019. Available online: (accessed on 19 June 2021).
