An extraction technology works on the principle of two consecutive steps that involves mixture of solute with solvent and the movement of soluble compounds from the cell into the solvent and its consequent diffusion and extraction. The conventional extraction techniques are mostly based on the use of mild/high temperatures (50–90 °C) that can cause thermal degradation, are dependent on the mass transfer rate, being reflected on long extraction times, high costs, low extraction efficiency, with consequent low extraction yields. Due to these disadvantages, it is of interest to develop non-thermal extraction methods, such as microwave, ultrasounds, supercritical fluids (mostly using carbon dioxide, SC-CO2), and high hydrostatic pressure-assisted extractions which works on the phenomena of minimum heat exposure with reduced processing time, thereby minimizing the loss of bioactive compounds during extraction. Further, to improve the stability of these extracted compounds, nano-encapsulation is required. Nano-encapsulation is a process which forms a thin layer of protection against environmental degradation and retains the nutritional and functional qualities of bioactive compounds in nano-scale level capsules by employing fats, starches, dextrins, alginates, protein and lipid materials as encapsulation materials.
1. Introduction
Bioactive compounds also known as secondary metabolites are widely present in plant matrix and over the past few decades, several in vitro and in vivo reports including epidemiological, and cohort studies provide evidence that consumption of plant-based food provides protection against several diseases. These bioactive extracts are also capable of treating chronic diseases including cancer, cardiovascular and diabetes mellitus (DM). Nutraceutical and pharmaceutical sectors use these extracts to develop functional food- and plant-based medicines, which have a potential to cure and deliver health benefits. According to the World Health Organization (WHO), about 80% of the global population depends on natural medicines. The initial steps followed to use these active compounds from plant matrix are extraction followed by pharmacological testing, isolation, characterization and clinical evaluation. Figure 1 represents a detailed flow chart of bioactive compound extraction from plant matrix.
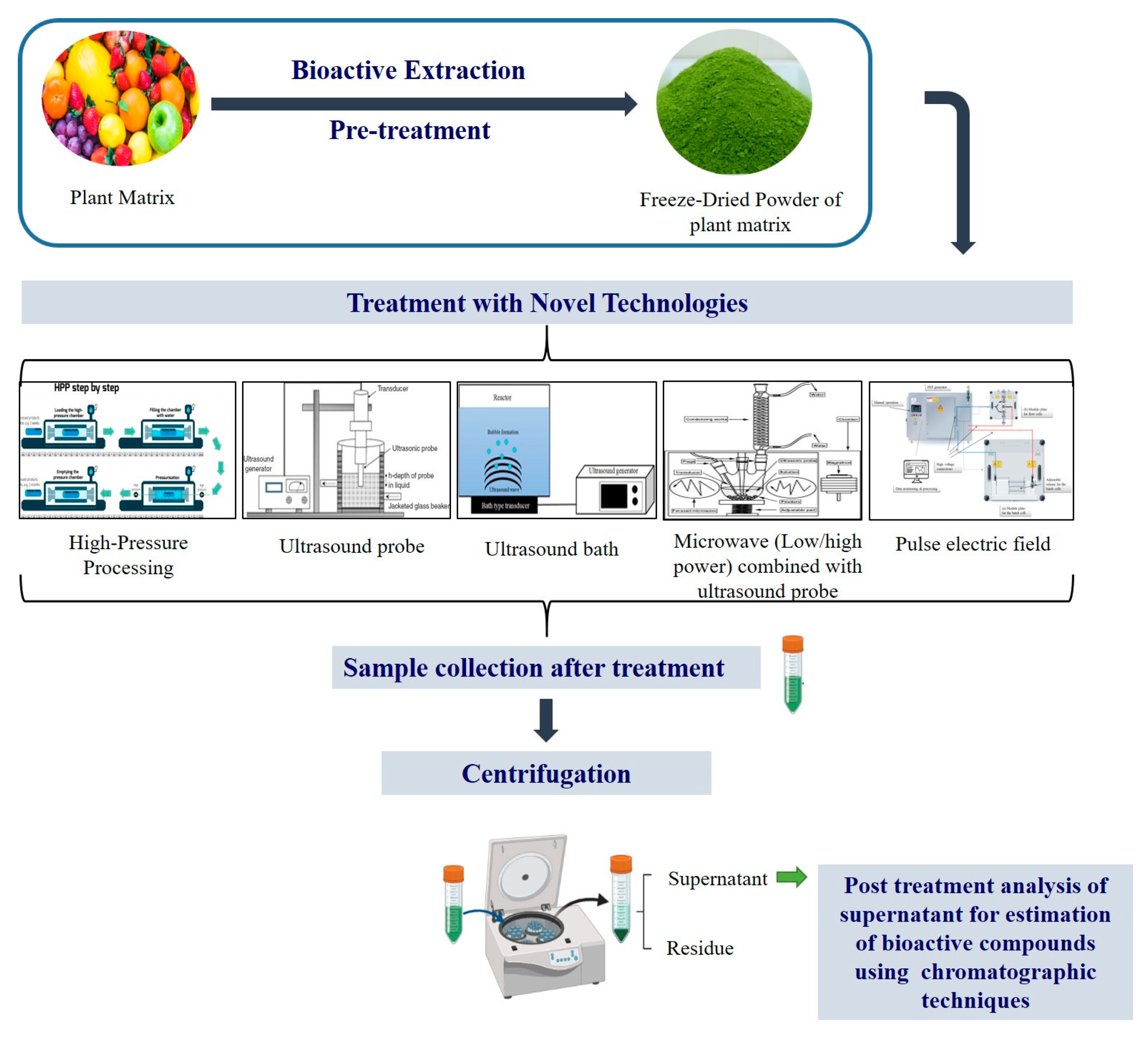 Figure 1.
Figure 1. Illustration for the extraction of bioactive compound using novel strategies.
The quality and yield of the bioactive compounds depend on two important factors: (a) the method opted for its extraction, (b) its extraction parameters including plant matrix type, solvent used, time and temperature. The most conventional method employed for bioactive extraction is Soxhlet extraction, maceration and hydro-distillation. Although, these techniques are commercially employed, but excessive use of solvents and longer processing times are the downsides of these technologies. Presently, demand for sustainable, chemical-free, advanced extraction processes with enhanced overall yield of bioactive compounds, also known as “green techniques”, which include ultrasound-assisted, enzyme-assisted, microwave-assisted, pulsed electric field-assisted, high-pressure processing, supercritical fluid and pressurized liquid extraction processes are gaining attention. Treating the plant matrix with these green technologies helps in breaking the cell structure, which allows the bioactive compound to leach or rinse out from the cell wall through solvents; as a result, it enhanced extraction yield. Further, purification of the extracted bioactive poses another technological challenge as each of these compounds has a unique molecular structure depending on their type, source and biological activity. The extracted compounds can be further purified, employing super critical CO
2 isolation, by addition of a co-solvent including ethanol, water to isolate the respective bioactive compound efficiently at an optimized temperature and pressure [
1]. In addition, it is essential to protect the extracted bioactive compound post extraction and purification, as these compounds are highly sensitive to environment exposure including moisture and high temperature (sensitive under heat, light, oxygen). Therefore, protection techniques such as nanoencapsulation are used to ensure that biological activity of these compounds is preserved until they reach and perform their function at the targeted location in the human body.
Encapsulation plays a vital role in protecting the bioactive compounds from getting degraded. At present, there are two kinds of encapsulation including microencapsulation and nanoencapsulation. The reason why nanoencapsulation is preferred over microencapsulation is due to its nano-scale size, as the smaller the size of the capsules, the higher their bioavailability and their release can be modified and controlled in a better way comparatively. Nanoencapsulation provides a protective shield around bioactive compounds. It is a system where a suitable nano-carrier, resistant to enzymatic degradation especially in gastrointestinal tract including chitosan, zein, and alginate, are widely used to encapsulate bioactive compounds employing several delivery methods including association colloids, nano-particles, nano-emulsions, nano-fibers/nano-tubes, nano-laminates. The selection encapsulation method is based on two main factors: (a) nature of the core material; (b) nature of wall material including wall material size, thickness, solubility, permeability and its rate of delivery. Basically, these techniques are classified into three main genres including chemical (emulsion and interfacial polymerization), physical–chemical (emulsification and coacervation) and physical–mechanical methods (spray-drying/spray-cooling/spray-congealing/prilling, freeze-drying, electrodynamic methods and extrusion) [
2]. However, in certain cases, combinations of these techniques are practiced as in the case of emulsification; first using homogenization, the emulsions are prepared and later converted to dry powder state using spray-drying and/or freeze-drying techniques [
3]. Reports indicate that about 80–90% of flavor encapsulation is done using spray-drying, while 5–10% by spray-chilling, 2–3% by melt extrusion and ~2% by melt injection [
4]. Castro et al. [
5] reported electro-spinning encapsulation as a heat-free technique to encapsulate fragrance and flavor, which is extremely promising for heat-sensitive compounds.
To recapitulate, this chapter provides a comprehensive summary on several aspects of bioactive extraction using non-thermal technologies and its nanoencapsulation. A brief description on nano-carriers employed for encapsulation is also discussed along with the detailed description of their application in food systems. Various opportunities and future challenges are also outlined.
2. Bioactive Compounds from Plant Materials
Ever since the beginning of human existence, plants have always been a boon for living a healthy life, as they not only provide a healthy environment to live, but most importantly, they provide food and bioactive compounds for medicinal use. In the beginning, plants and plant-related foods were used as a source of food and nutrition; later, their medicinal properties were discovered, which were able to cure diseases. Vinatoru et al. [
6] reported that Egyptian papyruses extracted oil from coriander and caster and used it in several applications including medicine, cosmetics and as preservatives. Further, Paulsen et al. [
7] reported that, during the Roman and Greek era, herbal plants were used by several therapeutics. According to literature [
8], bioactive compounds comprise three different categories including terpenes/terpenoids, alkaloids and phenolics. Basically, the chemical structure of these three categories differs, as shown in
Figure 2, and maximum bioactive compounds extracted from plant matrix belong to the terpenoids family.
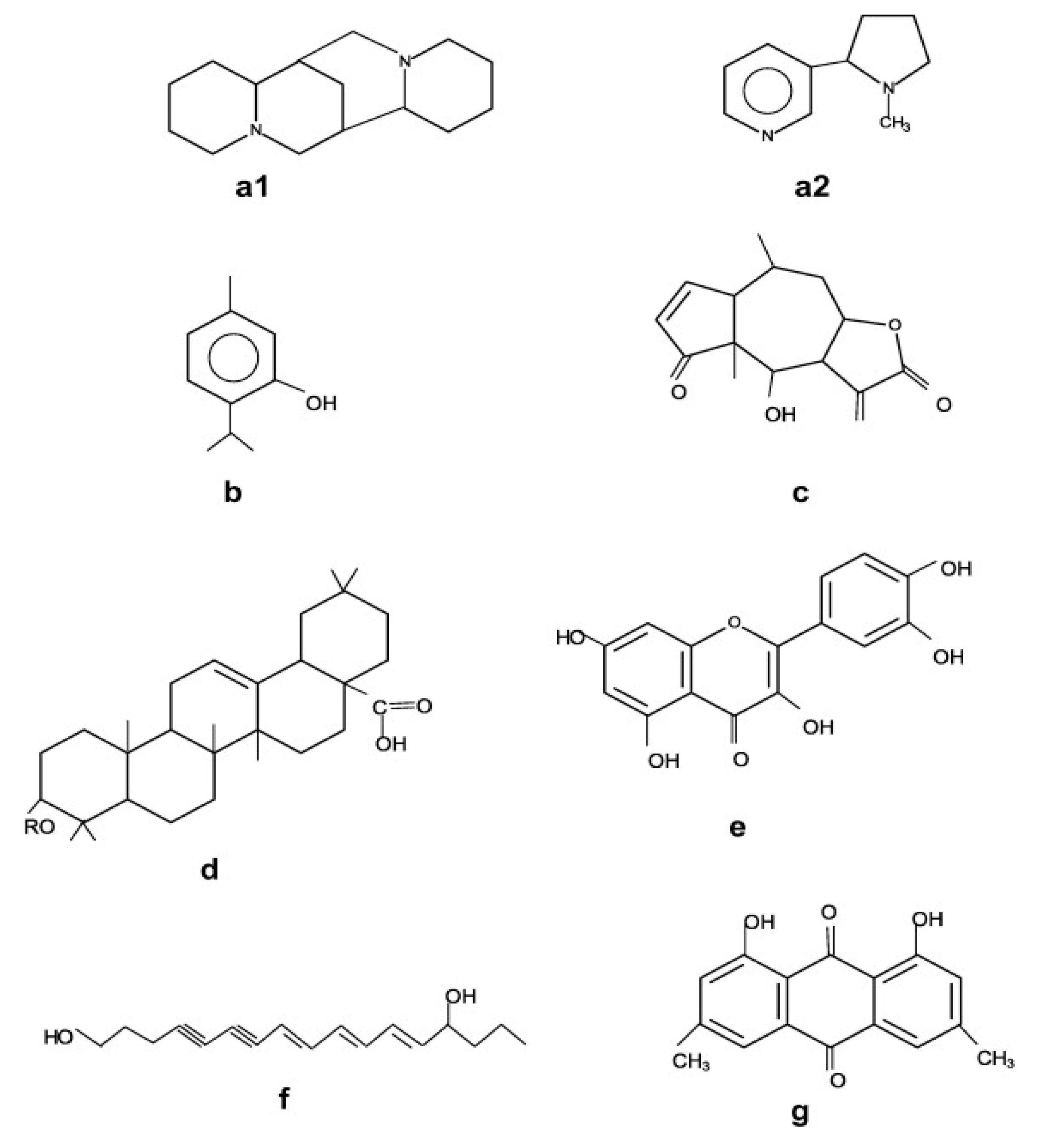 Figure 2.
Figure 2. Basic structures of plant bioactive compounds alkaloids (
a1,
a2), monoterpenes (
b), sesqueterpenes (
c), triterpenes, saponins, steroids (
d), flavonoids (
e), polyacetylenes (
f), polyketides (
g) (Adopted with permission from Wink et al. [
9].
Additionally, these compounds are classified based on their clinical and toxicological attributes as follows.
2.1. Glycosides
Glycosides are generally bonded by a mono/oligosaccharide or uronic acids. The part that is bonded with saccharide is called glycone and the other part is termed as aglycone, which consists of pentacyclic triterpenoids/tetracyclic steroids. The major subgroups of glycosides include cardiac glycosides, saponins, anthraquinone, glucosinolates and cyanogenics. Moreover, flavonoids commonly exist as glycosides. These glycosides are broken down in colon post ingestion; however, hydrophobic glycosides tend to get absorbed by the muscle cells. Cardiac glycosides are generally found in plants such as the Scrophulariaceae family, specifically in Digitalis purpura and in Convallaria majalis from Convallariaceae family. Additionally, cyanogenic glycosides can be found in the Prunus spp. of Rosaceae family as well as saponin, a bitter-tasting compound is found in glycosides. These saponin glycosides, found widely in the Liliaceas family (Narthesium ossifragum), is comprised of bigger molecules attached to hydrophilic glycone as well as hydrophobic aglycone, which creates forming quality, and thus it is used in the production of soap/detergent. Saponins also play an important role in modulating immune system and reducing blood sugar level. Besides, anthraquinone glycosids found in the Rumex crispus and Rheum spp. of polygonaceae family help in electrolyte secretion as well as induction of water and peristalisis in colon. Moreover, flavonoids are comprised of tri-ring at the center of the structure and proanthocyanidin is an oligomer in flavonoids. These two groups of compounds can also exist as glycosides. These are responsible for liberating antioxidant properties, inflammation and anti-carcinogenic activities. They are also responsible for the pigments in a wide range of plants. In addition, isoflavones are considered as a nutritional supplement type of bioactive molecules produced almost exclusively by the Fabaceae (Leguminosae or bean) family. They are basically a precise group of molecules, popularly known as phytochemicals, natively found in legumes and spices such as red clove. They are also considered as antioxidant molecules, as they help in the reduction of damage caused by oxygen in the body. Additionally, it plays a vital role in fighting against cancer cells.
2.2. Tannins
Tannins are widely found in plants, especially in the Fagaceae and Polygonaseae family. They are basically divided into two types, including condensed and hydrolysable tannins. Condensed groups of tannins are comprised of bigger polymers of flavonoids, while hydrolysable groups of tannins are clusters of monosaccharide (glucose) bonded with various derivatives of catechin. Tannin molecules tend to indiscriminately bind with protein molecules. Larger groups of tannins are used as medicine for treating skin bleeding, diarrhea and transudates.
2.3. Mono/Sesqui-Terpenoids and Phenylpropanoids
Synthesis of terpenoids takes place by a penta-carbon isoprene. In case of mono-terpenoids, two units of isoprene are found, while in sespui-terpenoids there are three units of isoprene. They are popularly known for their low molecular weight and wide range of categories (more than 25,000). However, phenylpropanoids comprises group of molecules where the basic carbon skeleton starts from nine and more, with strong odor, flavors and are volatile in nature. Generally, these compounds are commonly called volatile oils, widely found in the Lamiaceae family. It is used as an herbal medication including antineoplastic, antiviral and antibacterial effects. Besides, it also helps in gastrointestinal stimulation. In addition, diterpenoids are lipophilic non-volatile (odorless) compound and is a cluster of 4 units of isoprene with a strong flavor. It is widely found in various plants including Coffee arabica and popularly known for its antioxidant qualities.
2.4. Resins
Resins are composite mixtures which comprise both volatile as well as non-volatile attribute compounds; as well, they are comprised of a lipid soluble group of compounds. Non-volatile resins consist of diterpenoid and triterpenoid compounds, while volatile resins are equipped with mono/sesquiterpenoids. These resins are broadly found in herbaceous plants and are popularly known for their wound healing and antimicrobial properties.
2.5. Alkaloids
Alkaloids, a bitter-tasting and nitrogen-holding compound is a heterocyclic with limited spread in the plant kingdom. The Solanaceae family, including Atropa belladonna, Datura spp as well as Hyoscyamus niger, consists of tropane alkaloids with anticholinergic properties. It is widely used for reducing muscle pain. Besides, pyrrolizidine alkaloids belong to the Asteraceae and Boraginaceae family, especially in Senecio spp. It comprises of a wide range of application including treating cancer cells, stimulating bone marrow leucocytes and myocardial contractility. In addition, methylxanthine alkaloids are distributed in Coffee arabia as well as Theobroma cacao.
2.6. Proteins
Plant proteins have gained significant popularity in the field of food and medicinal sectors as they are a major source of nutrient for humans and animals. The Euphorbiaceae family as well as Fabaceae and lentils are known to contain a high content of protein.
3. Novel Strategies for the Extraction of Bioactive Compounds from Plant Matrix
3.1. Individual Strategies
3.1.1. Ultrasound-Assisted Extraction (UAE)
Bioactive extraction from plant matrix using ultrasound has been widely employed over the past few decades [
10]. It works on the principal of mechanical wave with a frequency ranging from 20 kHz to 100 MHz, which passes through a medium at a cycle of expansion and compression. In the case of liquid medium, cavitation bubbles are formed, at high acoustic pressure [
11]. This phenomenon is known as “acoustic cavitation” as it enhances the extraction yield as the high shear force is induced by the cavitation, which leads to mass transfer of bioactive compounds by turbulent mixing and acoustic flow [
12,
13]. Ultrasound (US) extraction works on four basic parameters including ultrasound power, ultrasonic intensity, mode of working (e.g., non-pulsed/pulsed) and acoustic energy density [
14]. In addition, it is divided into two different set-ups such as the US-bath and US-probe systems. In the case of an ultrasound bath system, the ultrasonic transducer array is placed below at the bottom of the extraction bath, which can also be attached at the side walls of the US-bath or inside the bath as a transducer array box. The transducer array box can be placed at any direction as per the requirement based on sample matrix. Whereas, in the case of the US-probe system, the probe is bonded with the transducer, which is submersed in liquid medium, enabling direct distribution of US waves, hence resulting in minimum loss of US energy. In addition, US intensity is an important factor affecting the yield of bioactive extraction, hence, it is important to consider the type of US employed, especially the probe diameter and the design of transducer employed as per the requirement [
15].
Over the past few years, US has evolved, as from the fixed US power system, now it is possible to adjust the acoustic power. Most probe-type US devices usually control the amplitude of the probe vibration, and some of them can apply busters to increase its maximum amplitude. Alexandru et al. [
16] developed a continuous US system for scale-up extraction to industrial level. In this system, a huge capacity of samples can be extracted continuously by feeding into a relatively small tank with multi-horn ultrasonic reactor. Elevated amplitude/intensity can enhance the sonochemistry but it leads to degradation of the transducer, which leads to increased agitation and reduced cavitation level. Hence, high amplitude/intensity is not essential to improve the extraction efficiency of cavitation level. However, the sample with high viscosity needs high amplitude as the high viscous samples tend to decrease the effect of sonication or cavitation [
17]. Therefore, to achieve the required level of cavitation, it is necessary to enhance the level of amplitude [
18]. Further, enhanced US frequency results in reduction in cavitation level. US develops cavitation bubbles, which take time to be initiated after the compression–rarefaction cycles. At high frequencies, it is challenging to produce acoustic cavitation, as the cycles of compression–rarefaction are too short to allow the growth of the cavitation bubbles. Therefore, higher amplitude and intensity of ultrasonic devices are needed to produce acoustic cavitation at high frequency [
19]. Apart from physical properties, chemical properties including solubility and stability of the target compound in selected solvent also play an essential role in the extraction yield and efficiency of bioactive compounds from plant matric using US. Parameters like time and temperature are also considered to influence the level of extraction [
20,
21].
Several bioactive compounds have been effectively extracted using US from a plant-based matrix (fruits, vegetables/their by-products) [
22,
23,
24]. Pan et al. [
25] extracted bioactive compounds from pomegranate peel using conventional and US extraction techniques. The results indicate that a continuous US-pulsed system enhanced the level of antioxidant extraction to 22–24% and reduced the extraction time to 90%. Due to the enhanced extraction yield and reduced time as well as energy consumption, US is considered as an alternate and green technology for the extraction of bioactive compounds. Apart from fruits and vegetables, US is also employed to extracted bioactive compounds from medicinal herbs, spices and oleaginous seeds [
26,
27,
28,
29].
3.1.2. Microwave-Assisted Extraction (MAE)
Over the past few years, microwave (MW) has gained popularity in the extraction of bioactive compounds from plant-based matrix [
30,
31,
32,
33]. It works on the principal of electromagnetic waves and frequencies, mostly 915 MHz and 2450 MHz frequencies employed for industrial and domestic applications. The mechanism behind this technique is that MW works on heating effect, which results in higher extraction temperature, causing faster mass transfer [
34]. MW has the tendency to penetrate inside the sample matrix causing interaction in polar components, thus causing direct/bulk heating effect to the solvent and the sample matrix [
12]. Further, this direct/bulk heating by MW also helps in the reduction of time and solvent used especially in industrial level extraction. Moreover, in MW extraction, the direct heating enters inside the matrix and increases local temperature and pressure, leaching out the target bioactive compounds from the sample matrix to the solvent solution. Two different setups are available for MW extraction: (a) open system, (b) closed system, where the pressure can be adjusted, e.g., increase or set at atmospheric level, respectively. The closed system MW extraction is carried out in a sealed vessel with constant MW heating, at controlled pressure and temperature. This closed system helps in reaching higher temperatures than the open system, as the amplified pressure in the closed vessel increases the boiling point of the extraction solvent [
35]. Although high temperature and pressure lead to efficient, high and fast extraction yield with less solvent consumption, they also escalate the safety risks (
Figure 3). In addition, this particular system is limited to certain bioactive compounds as maximum compounds are heat-sensitive and tends to degrade at elevated temperature, thus, the open system is promoted widely [
36].
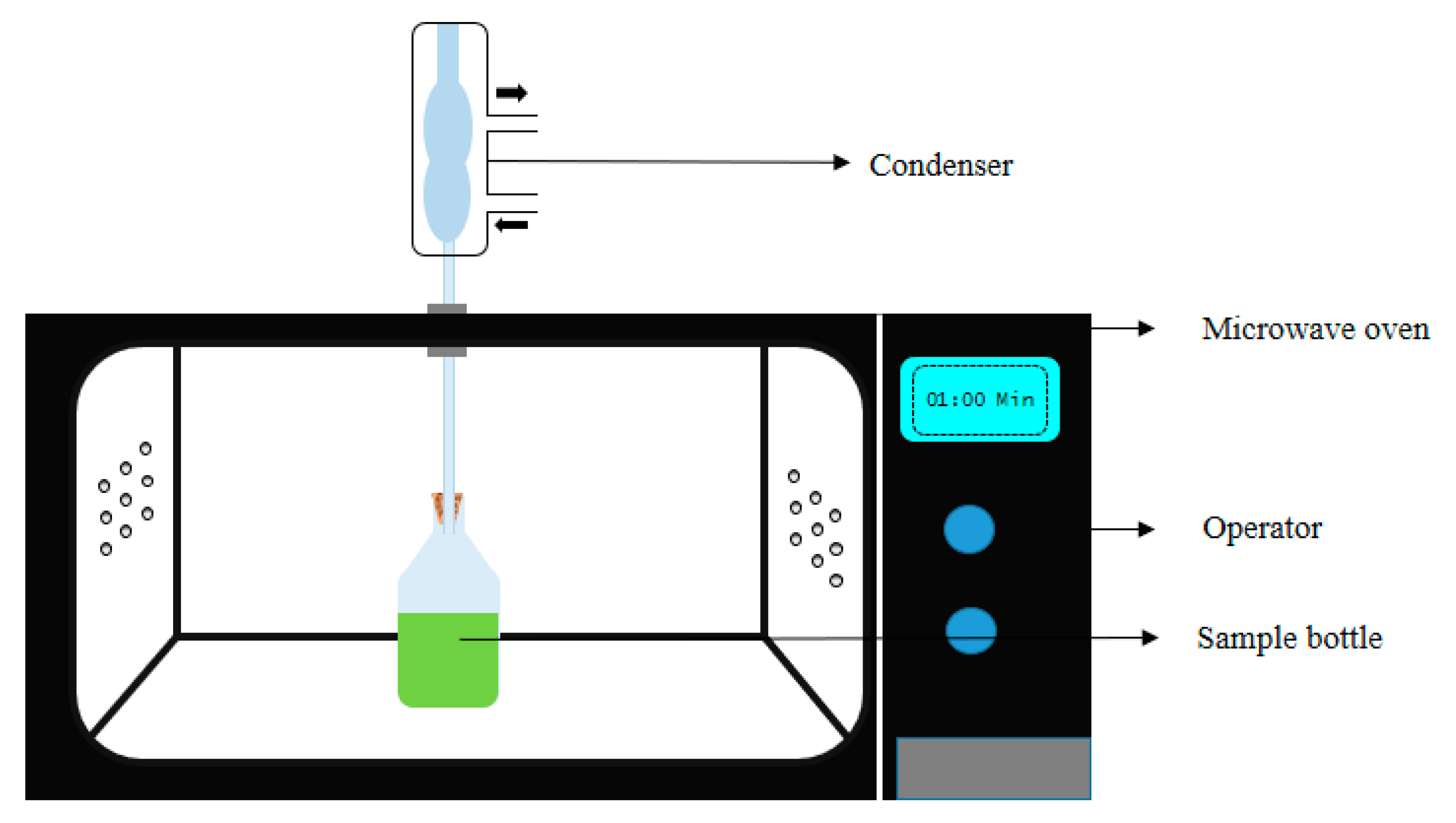 Figure 3.
Figure 3. Schematic representation of the microwave-assisted extraction apparatus.
MW extraction depends on several ranges of factors including microwave power, frequency, exposure time, moisture content, particle size of sample matrix, type and composition of solvent, dilution ratio, extraction temperature, extraction pressure and the number of extraction cycles. The detailed description of these factors has been reported by some reviews. The most critical factor is selection of extraction solvents as its solubility with sample matrix, its dielectric constant and dissipation plays a major role in extraction process. Solvents with higher dielectric constant like water and polar solvents can store more microwave energy than nonpolar solvents, thus water and polar solvents are reported to be better for MW extraction [
37]. Besides, the dissipation factor, which converts electromagnetic energy into heat, is considered a significant factor for MW extraction. Ajila et al. [
38] reported that solvents including ethanol and methanol, which contain a higher dissipation factor, are better solvents than water in the extraction of phenolics. Although, water possesses a high dielectric constant as compared to ethanol and methanol, but due to its low dissipation factor, it fails to heat up the sample matrix in depth. Therefore, for this reason, combination of solvents (water with ethanol or methanol), which contains high dielectric constant as well as a high dissipation factor, can be used to enhance the extraction efficiency.
MW extraction comprises a wide range of advantages over conventional extraction techniques including reduction in extraction time, solvent used and extraction cost with a significant level of enhancement in extracted compounds. Shu et al. [
39] extracted ginsenosides from ginseng root using MW extraction techniques for 15 min and resulted in a higher yield compared to conventional extraction, which took 10 h to complete. Dhobi et al. [
40] extracted flavolignin and silybinin from Silybum marianum employing MW extraction and results revealed that the extraction efficiency was enhanced to 60% compared with the conventional solvent extraction methods. Similarly, Asghari et al. [
41] extracted cinnamaldehyde and tannin from some medicinal Asian plants and their results reflected in a quicker and easier technique compared with conventional extraction techniques.
3.1.3. Enzyme-Assisted Extraction (EAE)
EAE is considered as one of the most efficient, eco-friendly, and non-thermal extraction strategies over conventional extraction techniques. It has been employed by several food industries for the extraction of various bioactive compounds saponin, carotenoid, anthocyanin and many more [
41]. Incorporation of certain enzymes including pectinases, cellulases and hemicellulases during extraction can significantly enhance extraction efficiency/yield of bioactive compounds by the principal of degradation in cell wall and membrane interiority [
42,
43]. In this technique, adequate knowledge about the catalytic specificity and its mode of action is necessary to acquire as well as to know its optimum conditions suitable for the enzymes to act on the plant matrixes. In order to use enzymes efficiently for EAE, it is essential to understand their catalytic specificity and mode of action, as well as investigate optimal conditions and which enzyme or enzyme combination is more suitable for the raw materials [
44]. Several significant factors including enzyme composition and concentration, type of extraction solvent, solid-to-liquid ratio, enzyme/substrate ratio, pH, extraction temperature and time play a vital role in activation of enzyme reaction and extraction of bioactive compounds [
12,
45].
Temperature is one of the important factors influencing the rate of extraction, but excessive increase of temperature may also inactivate enzymes. Moreover, a wide range of compounds are heat-sensitive, and therefore require mild temperature throughout the extraction process [
46]. Furthermore, pH is one of the major reaction conditions where the enzyme gets activated and stated to degrade the cells of the sample matrix. The optimum pH for each enzyme has already been reported; however, it may vary depending upon the matrix used and reaction conditions applied [
19,
47]. Enhancement in ratio between enzyme/substrate tends to improve catalytic reaction rate, but due to this amount of enzyme used, increases, which results in increased extraction cost. Further, the solvent used for the extraction may not be suitable for the enzymes used in the extraction process, for instance, several enzymes used for extraction of bioactive compounds from plant matrix are active in water, which may be not be active at higher concentrations of solvents including methanol and ethanol. Many bioactive compounds are highly soluble in concentrated methanol and ethanol; therefore, in such cases, major concern needs to be given to the selection of enzyme and its reaction conditions to achieve the desired extraction yield.
EAE is broadly employed for the extraction of bioactive components from a wide range of plant matrix including flavonoid from the peel of citrus, fructans from Dasylirion wheeleri, curcumin from turmeric, anthocyanin from beetroot, total phenolics from pomegranate peels and Cassia fistula pods, polysaccharide from seaweed and Cedrela sinensis, lycopene from tomato tissues and fatty acids from microalgae [
48,
49,
50,
51,
52,
53].
3.1.4. Pulse Electric Field-Assisted Extraction (PEF)
Over the years, PEF has become one of the most promising non-thermal and cost-effective bioactive extraction techniques to extract compounds used by the nutraceutical and pharmaceutical sector. The concept of PEF began in 1999 by a researcher named Ganeva and co-workers. They treated beer yeast with 2.75 kV/cm pulse electric power and kept it for macerating for 5 h for the extraction of protein. Further, they found that, after treating the sample with PEF, the dissolution enhanced, which was reflected in a significant increment in protein extraction. This gave a light of hope to the other researchers, as PEF has the ability to improve the mass transfer rate through cell membrane. Based on this belief, many researchers carried out several experiments for the extraction of bioactive compounds employing PEF and found that this non-thermal technology, when compared with several other extraction techniques, indicated shorter treatment time (some microseconds) with higher extraction yield [
54]. In addition, this technique can easily be employed at the industrial level for continuous flow of extraction. The system is equipped with a high-voltage pulse generator, a sample holding chamber and a controller. The treatment chamber contains two electrodes with a gap. One of the electrodes is connected to the pulse generator and the other is earthed (
Figure 4). Before treating the sample, it is subjected to fine powder, followed by agitation in the selected solvent. After agitation, the sample is pooled in the treatment chamber, led by the treatment parameter settings such as pulse number or pulse width (μs), electrode voltage (24 kV), energy input (kJ), frequency (Hz). Usually, at high electric voltage, the extraction is higher, but in the case of some compounds such as polysaccharides, it tends to decompose, which results in low extraction yield [
54]. Hence, it is important to decide the parameters of the extraction based on the targeted compounds. In addition to this factor, including solvent conductivity, polarity, solubility with the target compounds also plays a vital role in the extraction process. Generally, conductivity, solubility of the solvent and dilution ratio of solvent to solute as well as pulse duration/width is directly propositioning to the extraction yield. However, if we further increase these conditions beyond the requirement, then it tends to reflect negative results as when a high amount of electric pulse is applied, the targeted compound may also start to degrade, which can reduce the extraction yield and thus selection of the correct solvent, and operation factors are extremely significant [
55]. Bioactive compounds including protein, saccharides, calcium and others are easily extracted using PEF.
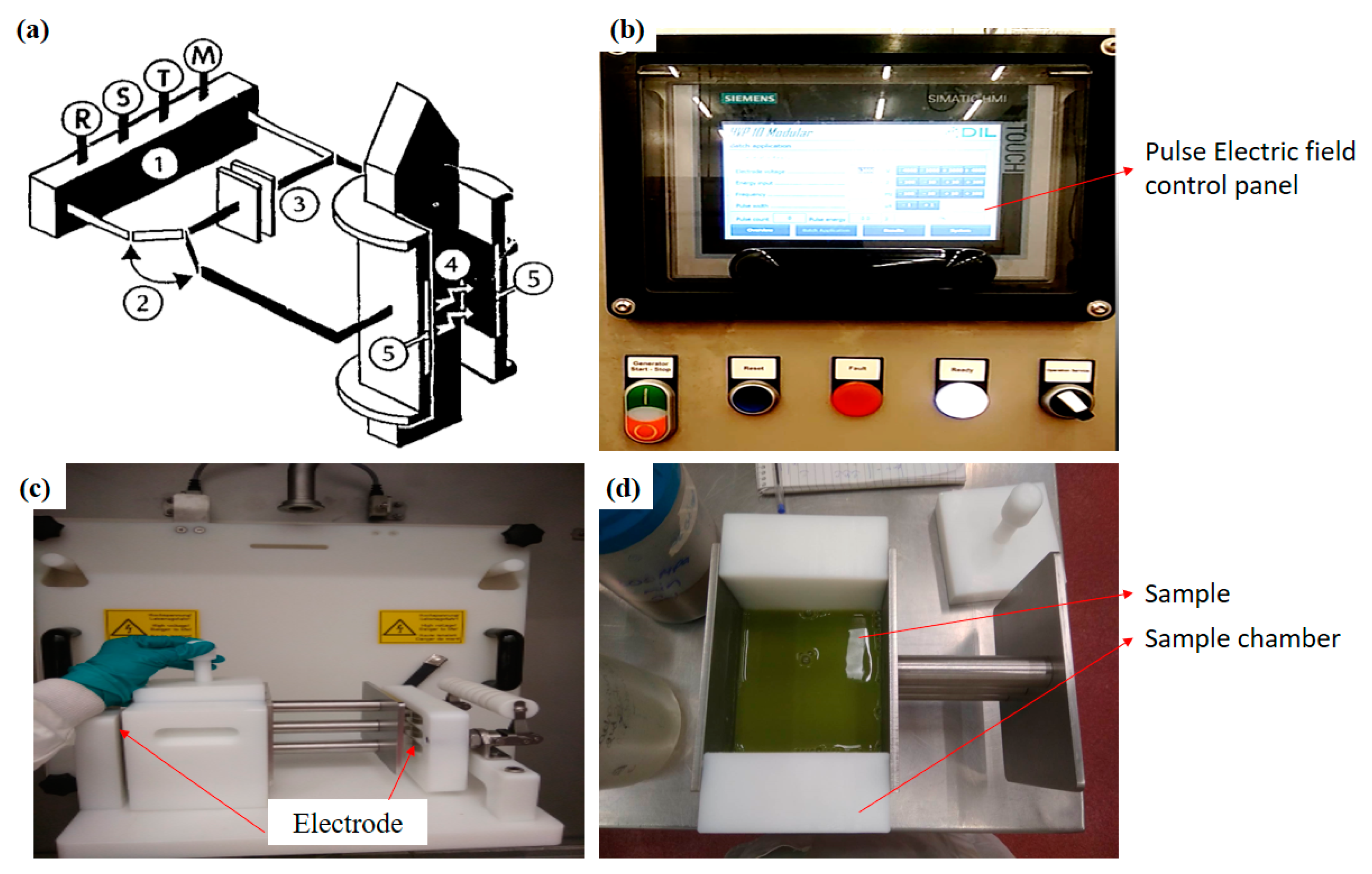 Figure 4.
Figure 4. Simplified schematic diagram of PEF (
a) 1. High-voltage generator; 2. Switch; 3. Capacitor; 4. Medium; 5. Electrodes: and R, S, T, M, connector points for the main supply; (
b) control panel of PEF; (
c) Pulse electric field electrodes and treatment chamber; (
d) sample chamber with sample. (Modified and adopted with permission from Knorr et al. [
56].
3.1.5. Moderate Pressure Application
 Figure 1. Illustration for the extraction of bioactive compound using novel strategies.
Figure 1. Illustration for the extraction of bioactive compound using novel strategies. Figure 2. Basic structures of plant bioactive compounds alkaloids (a1,a2), monoterpenes (b), sesqueterpenes (c), triterpenes, saponins, steroids (d), flavonoids (e), polyacetylenes (f), polyketides (g) (Adopted with permission from Wink et al. [9].
Figure 2. Basic structures of plant bioactive compounds alkaloids (a1,a2), monoterpenes (b), sesqueterpenes (c), triterpenes, saponins, steroids (d), flavonoids (e), polyacetylenes (f), polyketides (g) (Adopted with permission from Wink et al. [9]. Figure 3. Schematic representation of the microwave-assisted extraction apparatus.
Figure 3. Schematic representation of the microwave-assisted extraction apparatus. Figure 4. Simplified schematic diagram of PEF (a) 1. High-voltage generator; 2. Switch; 3. Capacitor; 4. Medium; 5. Electrodes: and R, S, T, M, connector points for the main supply; (b) control panel of PEF; (c) Pulse electric field electrodes and treatment chamber; (d) sample chamber with sample. (Modified and adopted with permission from Knorr et al. [56].
Figure 4. Simplified schematic diagram of PEF (a) 1. High-voltage generator; 2. Switch; 3. Capacitor; 4. Medium; 5. Electrodes: and R, S, T, M, connector points for the main supply; (b) control panel of PEF; (c) Pulse electric field electrodes and treatment chamber; (d) sample chamber with sample. (Modified and adopted with permission from Knorr et al. [56].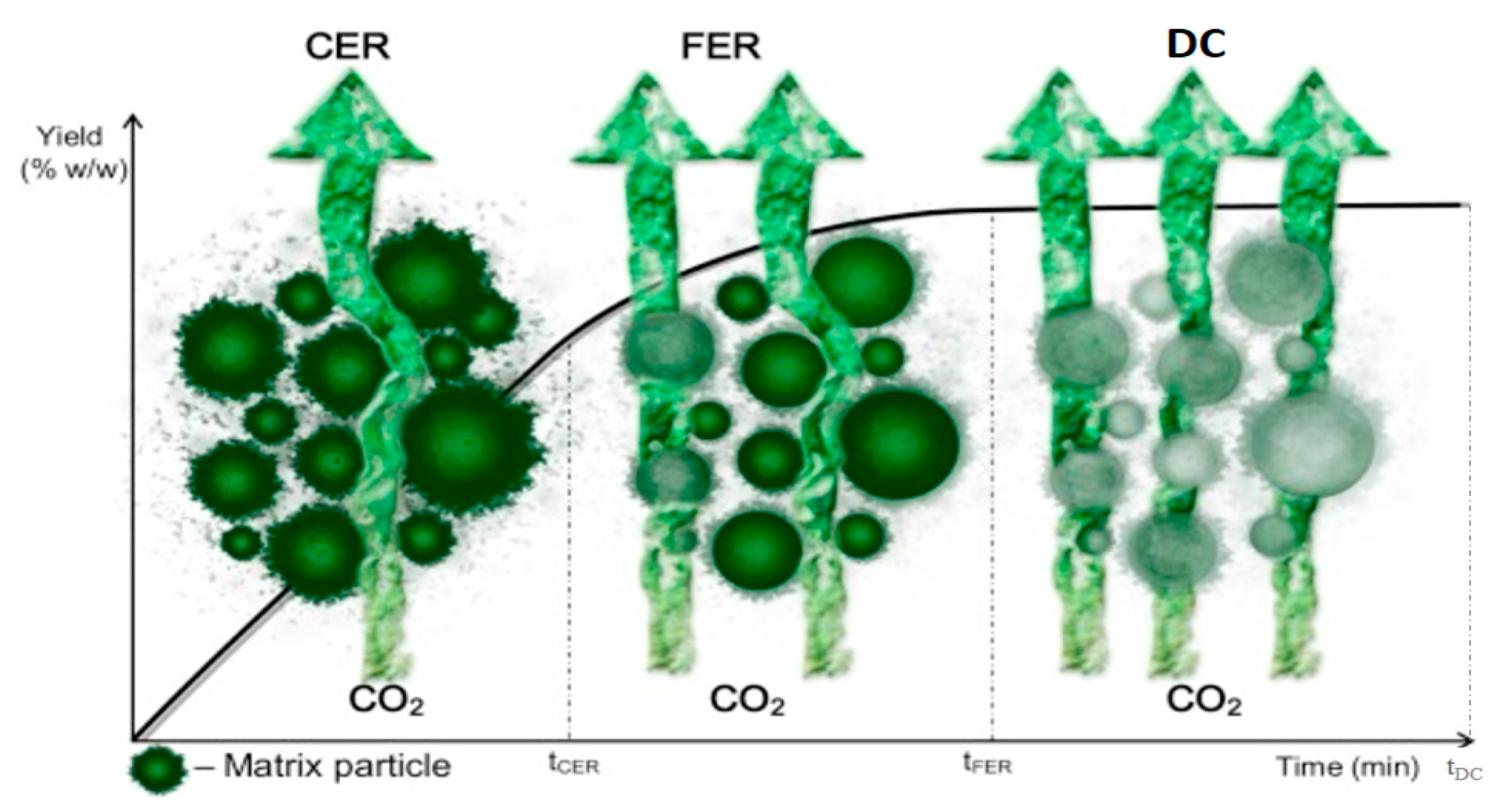 Figure 5. Graphical representation of mass transfer of bioactive compounds from cell membrane to solvent (modified and adopted with permission from Rui et al. [62]).
Figure 5. Graphical representation of mass transfer of bioactive compounds from cell membrane to solvent (modified and adopted with permission from Rui et al. [62]).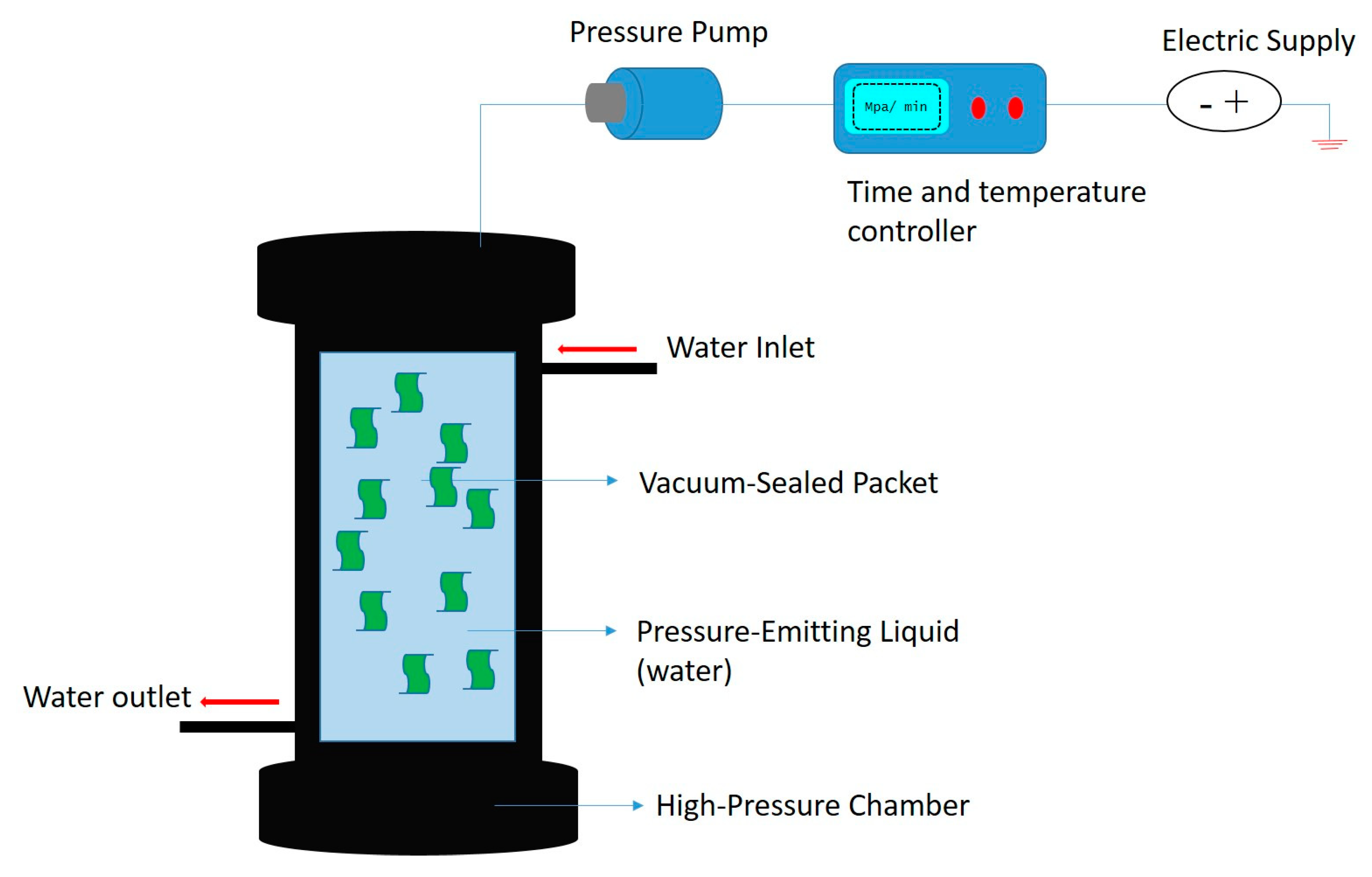 Figure 6. Schematic representation of high-pressure processing.
Figure 6. Schematic representation of high-pressure processing.