Bone morphogenetic proteins (BMPs) were originally identified as the active components in bone extracts that can induce ectopic bone formation. In recent decades, their key role has broadly expanded beyond bone physiology and pathology. Nowadays, the BMP pathway is considered an important player in vascular signaling. Indeed, mutations in genes encoding different components of the BMP pathway cause various severe vascular diseases. Their signaling contributes to the morphological, functional and molecular heterogeneity among endothelial cells in different vessel types such as arteries, veins, lymphatic vessels and capillaries within different organs. The BMP pathway is a remarkably fine-tuned pathway. As a result, its signaling output in the vessel wall critically depends on the cellular context, which includes flow hemodynamics, interplay with other vascular signaling cascades and the interaction of endothelial cells with peri-endothelial cells and the surrounding matrix.
- BMP
- BMP pathway fine-tuning
- lymphatic vessel biology
- mechano-transduction
- vascular malformations
- signaling cross-talk
1. Introduction
2. Fine-Tuning Mechanisms of BMP Signaling in the Vasculature
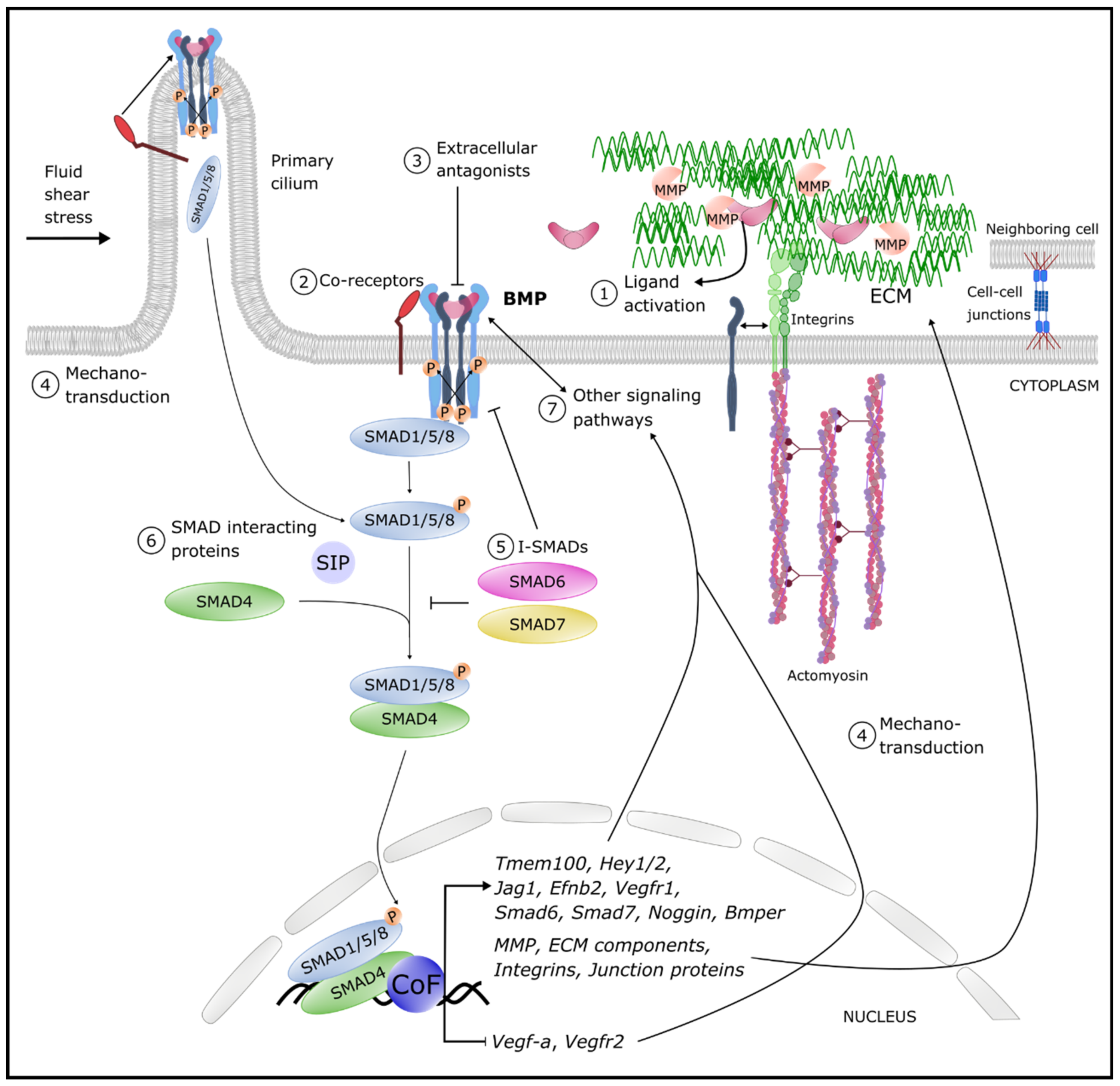 Figure 1. Overview of the different levels of BMP pathway fine-tuning. Circled numbers denote examples of levels of regulation of the signaling output. Cell–cell junctions are tight, adherence and gap junctions (details are provided in the text). Abbreviations: BMP: bone morphogenetic protein BMPER: BMP endothelial cell precursor-derived regulator; CoF: co-factors; P: Phosphorylation; ECM: extracellular matrix; Ephb2: Ephrin B2; Hey: hairy/enhancer-of-split related with YRPW motif protein; Jag: Jagged; MMP: Matrix metalloproteinases; SIP: SMAD interacting proteins; Tmem100: transmembrane protein 100; Vegf: vascular endothelial growth factor; Vegfr: VEGF receptor.
Figure 1. Overview of the different levels of BMP pathway fine-tuning. Circled numbers denote examples of levels of regulation of the signaling output. Cell–cell junctions are tight, adherence and gap junctions (details are provided in the text). Abbreviations: BMP: bone morphogenetic protein BMPER: BMP endothelial cell precursor-derived regulator; CoF: co-factors; P: Phosphorylation; ECM: extracellular matrix; Ephb2: Ephrin B2; Hey: hairy/enhancer-of-split related with YRPW motif protein; Jag: Jagged; MMP: Matrix metalloproteinases; SIP: SMAD interacting proteins; Tmem100: transmembrane protein 100; Vegf: vascular endothelial growth factor; Vegfr: VEGF receptor.3. BMP-Linked Vascular Pathologies
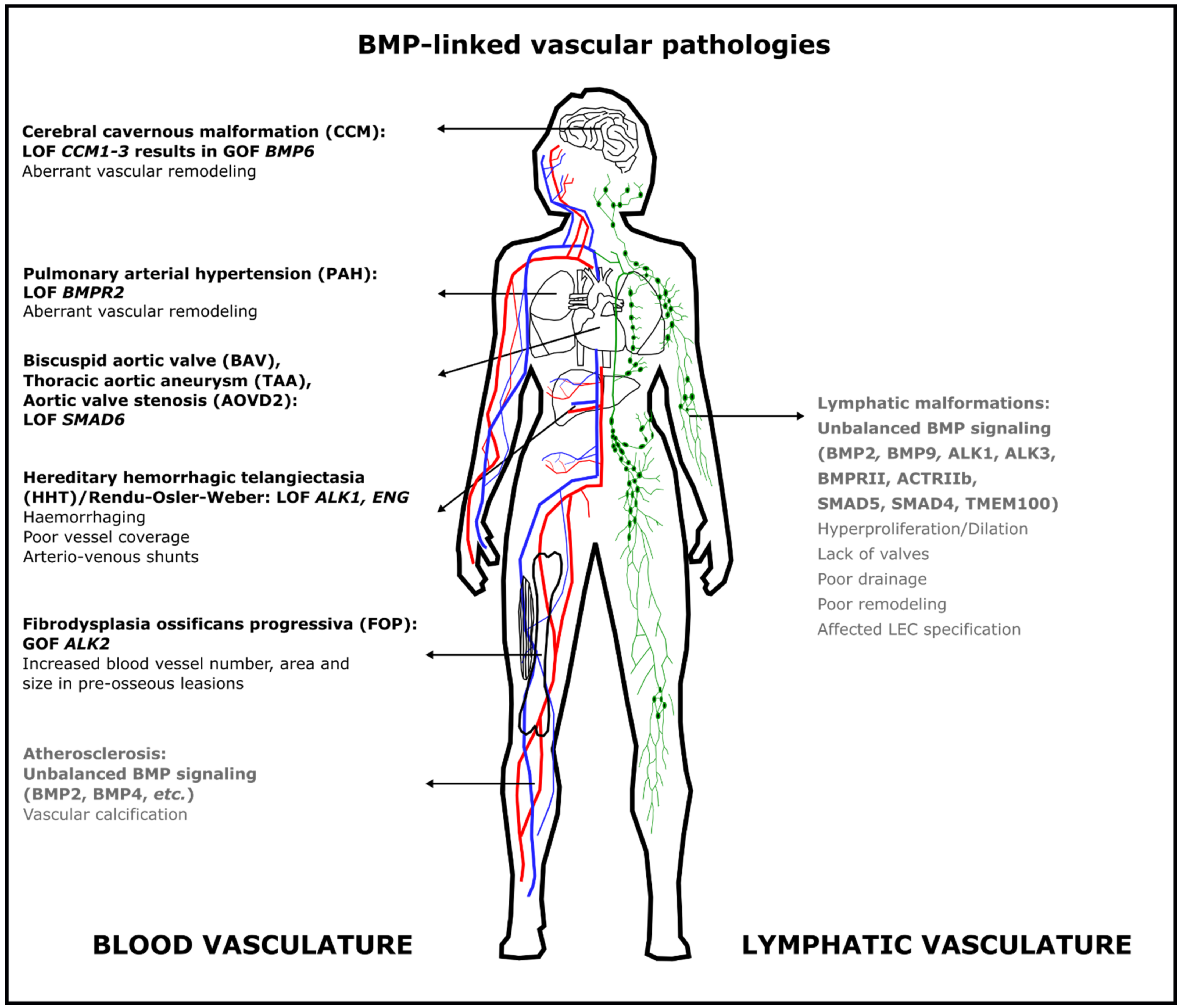 Figure 2. Aberrant BMP signaling in the blood vasculature causes different severe but rare diseases in humans. Some pathologies are due to loss of function of BMP signaling, whereas others result from gain of function of BMP signaling. The most frequent mutations are indicated here; additional mutations are discussed in the text. Text in gray indicates that this role for BMP signaling has only been demonstrated in animal models. Abbreviations: ACTRII: activin type II receptor; ALK: activin receptor-like kinase; AOVD2: aortic valve stenosis; BAV: bicuspid aortic valve; BMP: bone morphogenetic protein; BMPR2: BMP Type 2 receptor; CCM: cerebral cavernous malformation; ENG: endoglin; FOP: fibrodysplasia ossificans progressiva; HHT: hereditary hemorrhagic telangiectasia; GOF: gain of function; LEC: lymphatic endothelial cell; LOF: loss of function; PAH: pulmonary arterial hypertension; TAA: thoracic aortic aneurysm; TMEM100: transmembrane protein 100.
Figure 2. Aberrant BMP signaling in the blood vasculature causes different severe but rare diseases in humans. Some pathologies are due to loss of function of BMP signaling, whereas others result from gain of function of BMP signaling. The most frequent mutations are indicated here; additional mutations are discussed in the text. Text in gray indicates that this role for BMP signaling has only been demonstrated in animal models. Abbreviations: ACTRII: activin type II receptor; ALK: activin receptor-like kinase; AOVD2: aortic valve stenosis; BAV: bicuspid aortic valve; BMP: bone morphogenetic protein; BMPR2: BMP Type 2 receptor; CCM: cerebral cavernous malformation; ENG: endoglin; FOP: fibrodysplasia ossificans progressiva; HHT: hereditary hemorrhagic telangiectasia; GOF: gain of function; LEC: lymphatic endothelial cell; LOF: loss of function; PAH: pulmonary arterial hypertension; TAA: thoracic aortic aneurysm; TMEM100: transmembrane protein 100.4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms22126364
References
- García de Vinuesa, A.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2015.
- Goumans, M.-J.; Zwijsen, A.; ten Dijke, P.; Bailly, S. Bone morphogenetic proteins in vascular homeostasis and disease. Cold Spring Harb. Perspect. Biol. 2018, 10.
- Morrell, N.W.; Bloch, D.B.; Ten Dijke, P.; Goumans, M.J.T.H.; Hata, A.; Smith, J.; Yu, P.B.; Bloch, K.D. Targeting BMP signalling in cardiovascular disease and anaemia. Nat. Rev. Cardiol. 2016, 13, 106–120.
- Cunha, S.I.; Magnusson, P.U.; Dejana, E.; Lampugnani, M.G. Deregulated TGF-β/BMP signaling in vascular malformations. Circ. Res. 2017, 121, 981–999.
- Hiepen, C.; Mendez, P.-L.; Knaus, P. It takes two to tango: Endothelial TGFβ/BMP Signaling crosstalk with mechanobiology. Cells 2020, 9, 1965.
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105.
- Pulkkinen, H.H.; Kiema, M.; Lappalainen, J.P.; Toropainen, A.; Beter, M.; Tirronen, A.; Holappa, L.; Niskanen, H.; Kaikkonen, M.U.; Ylä-Herttuala, S.; et al. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis 2020, 24.
- Helbing, T.; Rothweiler, R.; Ketterer, E.; Goetz, L.; Heinke, J.; Grundmann, S.; Duerschmied, D.; Patterson, C.; Bode, C.; Moser, M. BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood 2011, 118, 5040–5049.
- Choi, E.J.; Walker, E.J.; Shen, F.; Paul Oh, S.; Arthur, H.M.; Young, W.L.; Su, H. Minimal homozygous endothelial deletion of eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc. Dis. 2012, 33, 540–547.
- Bernabeu, C.; Bayrak-Toydemir, P.; McDonald, J.; Letarte, M. Potential second-hits in hereditary hemorrhagic telangiectasia. J. Clin. Med. 2020, 9, 3571.
- Pachori, A.S.; Custer, L.; Hansen, D.; Clapp, S.; Kemppa, E.; Klingensmith, J. Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. J. Mol. Cell. Cardiol. 2010, 48, 1255–1265.
- Nakagawa, Y.; Ikeda, K.; Akakabe, Y.; Koide, M.; Uraoka, M.; Yutaka, K.T.; Kurimoto-Nakano, R.; Takahashi, T.; Matoba, S.; Yamada, H.; et al. Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1908–1915.
- Morikawa, M.; Mitani, Y.; Holmborn, K.; Kato, T.; Koinuma, D.; Maruyama, J.; Vasilaki, E.; Sawada, H.; Kobayashi, M.; Ozawa, T.; et al. The ALK-1/SMAD/ATOH8 axis attenuates hypoxic responses and protects against the development of pulmonary arterial hypertension. Sci. Signal. 2019, 12.
- Liu, T.; Zou, X.Z.; Huang, N.; Ge, X.Y.; Yao, M.Z.; Liu, H.; Zhang, Z.; Hu, C.P. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019, 227, 64–73.
- Tian, F.; Zhou, A.X.; Smits, A.M.; Larsson, E.; Goumans, M.J.; Heldin, C.H.; Borén, J.; Akyürek, L.M. Endothelial cells are activated during hypoxia via endoglin/ALK-1/SMAD1/5 signaling in vivo and in vitro. Biochem. Biophys. Res. Commun. 2010, 392, 283–288.
