Vegetable and ornamental crops require high input demand to adequately support their standard commercial quality and yield. For these crops, a very high level of agronomic use efficiency of many productive factors can be achieved in soilless culture. The challenges that we now face: (i) making soilless systems more inclusive of sustainable and eco-friendly growing substrates, possibly available at a local level; (ii) replacing chemicals with more sustainable products (e.g., organic active compounds) as much as possible for plant nutrition and protection. This may be addressed by different approaches, among which the adoption of peat-free organic substrates may play a central role.
- circular horticulture
- growing media
- ornamentals
- vegetables
- plant nutrition
- plant protection
1. Introduction
Soilless culture, defined as any method of growing plants without the use of soil as a rooting medium, is the most intensive and effective production method in the modern agriculture industry [1]. Nowadays, soilless cropping is carried out to produce vegetable and ornamental plants in greenhouses and nurseries. Such systems refer to a number of container growing systems, including those where plant roots grow in a rooting medium known as a “substrate” or “growing medium” [2]. In comparison to soil-based cultivation, soilless systems are characterized by higher initial investment costs. Nevertheless, these higher costs are justified by increased productivity per unit area [3], although reliable and long-term-based economic evaluations are rarely available [4]. Importantly, in the case of horticulture production, soilless culture is characterized by a more accurate control of plant nutrition and irrigation and other environmental conditions of the rhizosphere (e.g., root zone temperature). Also, it may drastically reduce the use of pesticides and fungicides, and it eliminates agrochemicals to control weeds. Finally, soilless cultivation has been observed to be an effective way to improve product quality, as in the case of food biofortification, which is even more important than yield, per se, to increase farm competitiveness in modern horticulture [5].
2. Soilless Systems and Circular Horticulture
The circular economy represents an economic model based on sharing, leasing, reuse, repair, refurbishment, and recycling in a (almost) closed loop, aiming at retaining the highest utility and value of products, components, and materials at all times [6].
Looking in detail at soilless closed systems, it is true that minimal substrate is required, reducing the problem of pollution of the environment from its disposal [1]. However, the drivers for the selection of growing media have until very recently been the combination of their performance and cost [2]. In fact, it is only in recent times that the attention has been moved to the reduction of the environmental impact of plant production via the selection of more eco-friendly substrates [7]. Substrates identified by environmental drivers are mainly composed of organic components [8]. Reasons are to be found among their general low cost and widespread availability, together with their renewability and easier disposal [9] that make them an environmentally sustainable option [2]. Among these, the organic materials used globally in soilless cultivation are primarily peat, coir, composted materials, and wood refuses [2]. However, the worldwide most used peat is facing several limitations [10][11]. Thus, the recent gradual peat replacement is driven by the attention on recycling organic wastes in an environmentally sensitive manner together with the rising cost of peat [9].
3. The Role of Organic Substrates in Plant Nutrition and Irrigation of Soilless Crops
3.1. Biochar
The presence of biochar can improve the physicochemical characteristics of soilless growing media due to the high porous structure, large surface area, high ion exchange, and water-holding capacities [12][13]. However, it must be noted that although many works agree on its possible contribution to plant nutrition, the results are not conclusive, and much more research is required due to the high variability observed in terms of plant yield and quality.
Biochar composition variability is mostly related to raw organic materials and temperature conditions in the pyrolysis process. In soilless-grown tomato, Petruccelli et al. [14] tested three biochar types obtained from different green refuses, but only in the case of poplar wood chips crop yield and quality were positively influenced; no effect was reported in the other cases. Suthar et al. [15] focused on how biochar obtained with a lower pyrolysis temperature induced a higher growth rate in tomatoes when added to the growing medium than that of biochar obtained with higher temperatures. The lower performance could be also related to the higher pH resulting from higher pyrolysis temperatures; this type of biochar induced lower N concentration in the soilless cultivation of endive plants [16].
The higher capacity of retaining NH4 and K [17][18] makes biochar a sustainable tool by which nutrient losses from soilless systems can be reduced—this is one of the main concerns in the application of soilless cultivation in southern Europe [4]—especially if combined with efficient irrigation management. The addition of biochar (25% v v−1) reduced the leachate volume, per irrigation event, and leaching fraction of container-grown wood plants in a timer-based irrigation system [19]. In contrast, the high water-holding capacity of some types of biochar may cause imbalanced micro- and macro-porosity of the growing medium with low drainage and reduced air exchanges in the root zone, which in turn could result in anoxia phenomena with reduced yield [20].
3.2. Coir
One of its chemical parameters that may vary in a broad range is pH (from 4.8 to 6.9), with values that are on average higher than in peat [17][8][21]. Due to the key role that pH plays in plant nutrition in soilless cropping, pH adjustments could be necessary [22]. Starting from the middle period of the cultivation cycle of potted blueberry, pH measured in the leachate of a coir-based substrate has been found to not considerably differ to that in a bark-based substrate [23]. The presence of essential and beneficial nutrients, EC (electrical conductivity) level, and particle size can also vary depending on the original material and processing [17][8][21][24]. Cation exchange capacity (CEC) is considered generally low in coir compared with other organic substrates (32–95 cmol kg−1) [24][25]. Another relevant parameter is the C/N ratio, which, in coir, is very high (75–184) due to its content in lignin and cellulose that can cause immobilization of soluble N. Additional N fertilizers could then be required to avoid shortages of this nutrient throughout the cultivation cycle [8][24][25]. When compared with other organic substrates, coir indeed results poor in N and P, which are often not detectable [17][8][24], but it is rich in K [17][8][21][24].
3.3. Green Compost
Focusing on the nutritional contribution of green compost, the elements’ mineralization rate and the resulted nutrients, in particular N release, are significantly positive aspects of crop production [9]. As previously mentioned, compost can contribute a part of crops’ nutritional needs [26], even if the exact nutrient levels together with their potential release rate are often unknown [9]. Moreover, results on this aspect are often highly variable and depend on the waste origin of the tested compost [27]. With respect to this, Table 2 reports the green compost concentration of N, P, and K according to different bibliographic sources.
| N | P | K | Source |
|---|---|---|---|
| (g kg−1 DW) | (g kg−1 DW) | (g kg−1 DW) | |
| 12.3–15.2 | 0.2–0.48 | 12.1–19.9 | [28] |
| 12.2 | 2.6 | 7.3 | [29] |
| 16.3 | Na | Na | [30] |
| Na | 1.8–2.5 | 5.6–11.1 | [31] |
| 15.4 | 3.1 | 8.6 | [32] |
Compost substrates can also improve water retention [33], i.e., for green roof decks where the addition of green compost increased water-holding capacity and as a consequence influenced water retention on the decks [34].
3.4. Wood Fiber
The major limiting factors in the use of fresh wood products as growing media are N fixation and the high content in potential phytotoxic compounds, such as phenols and tannins [35][36]. Composted or stabilized wood products are usually preferred as substrate component but not as stand-alone growing media because of their limited water-holding capacity [2][17]. Nevertheless, wood fibers substrates proved to be successful in the production of both vegetables [37] and woody ornamentals [38][39].
In an experiment investigating wood fibers as components of growing media for the fast-growing species Prunus laurocerasus, it was found that such substrate might impose constrains in water availability [40]. In fact, for sustainable irrigation management in container grown crops, the components inherent physical properties, and the number and size of pores of the chosen substrates play a critical role [41]. Nevertheless, the size and shape of the substrate components can be manipulated to decrease the proportion of large pores, thus potentially increasing the irrigation efficiency [42] and, consequently, nutrient management. Similarly, pore uniformity is as important as overall size; thus, such substrates can improve their performances in combination with different substrate amendments (e.g., biochar and compost), which would increase the water-holding capacity and, consequently, reduce the water requirement for high-value crops and mitigate water and nutrient leaching [43]. The addition of wood fiber to peat-based growing media has been proved to reduce substrate volume loss in tomato seedling and transplanting trials, thus improving plant growth [44][45].
4. The Role of Organic Substrates in Crop Tolerance to Abiotic and Biotic Stresses of Soilless Crops
4.1. Biochar
Salinity stress is one of the main investigated abiotic stresses that often occurs in soilless cultivation, especially in coastal areas where saline ions can infiltrate irrigation water [4]. A reduction in the detrimental effects of salinity, possibly occurring in the root zone of potted plants, has been related to the high Na adsorption ability of biochar that induces a low Na-to-K ratio in the xylem [48]. Awad et al. [49] observed a significant reduction in the accumulation of Na in the leaves of cabbage and dill grown on biochar than those grown on perlite. The addition of biochar to the growing media of ornamental shrubs limited the damage of salinity in Prunus laurocerasus irrigated with water containing NaCl at critical concentration for this species [50]. However, despite its capacity to reduce the impact of saline ions on plants, biochar itself may present higher EC compared with that of other organic substrates, which can vary from low to very high, depending on the feedstock material [17]. In general, biochar obtained from livestock litter has higher EC than that of green refuses due to the higher concentration in macrocations [51]. This higher EC can therefore represent a limit to the use of this material to grow plants that are sensitive to osmotic stress, such as many ornamental crops. On the other hand, this extra nutrient budget can be used to overcome nutritional impairments possibly occurring in the root zone or for the supply of nutrients as an alternative source to chemical fertilizers [52][53]. Biochar indeed shows higher pH compared with that of peat whose pH, conversely, must be raised before use as a substrate in most containerized crops. The simple expedient of prewashing and addition of natural or chemical acids would be successful in reducing both the pH and EC levels of biochar products, thus making the application of this material safe in the replacement of peat [54].
4.2. Coir
Coir can improve the physiological responses of plants to unfavorable external inputs. Its role in increasing plant resistance to abiotic stresses was, for instance, highlighted in broccoli crop grown on coir dust substrate, reaching a higher marketable yield compared to that of plants grown in perlite, both in normal and in low irrigation regimes [55]. In fact, the higher water-holding capacity of coir compared to that of perlite [21] enhances plant resistance to low irrigation conditions. Similarly, coir was found to increase water use efficiency in potted marigold (Tagetes erecta), and a positive response of the fiber amended plants to drought stress [56].
4.3. Green Compost
Since in organic media additional beneficial elements and bioactive compounds are released from the decomposing organic component, these substances could be responsible for enhanced plant growth and resistance to abiotic and biotic stresses [57]. The relation between green compost and compost-grown plants tolerance to abiotic stresses is strongly connected to the selection of the raw material used to obtain the compost [9][58]. Compost as a growing media can be responsible for the increased salinity in the root zone, with K and Na among the more easily extractable elements resulting in an increase. However, compost’s salinity can easily be reduced quite [9].
The bioactive molecules that are part of compost play a major role in enhancing plant resistance to abiotic stresses, having beneficial effects on plants and improving their capability to face adverse environmental conditions [59], even if the mechanisms activated are often difficult to identify and are still under investigation [60]. Humic substances in particular were found to stimulate plants root growth and development influencing nutrient uptake and root architecture [61], thus leading to a better uptake of water and nutrients as well as increased tolerance to environmental stresses [62].
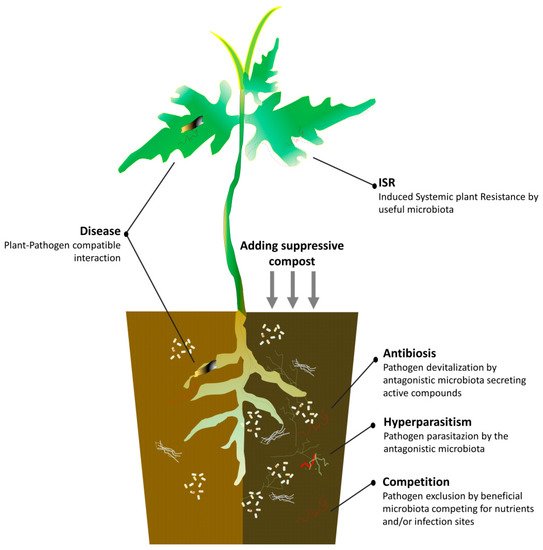
Green composts also have the greatest potential to impact plant health and development because of their disease-suppressive properties [63][64][65]. Aliquots of a quality agricultural waste compost incorporated as additional or basic component could contribute to better redesign of the suppressive profile of the ready-for-crop substrates and reduce the needs of disease management for chemicals [66][67]. Suppressiveness means that the compost is able to hinder the pathogenesis by interfering with at least one of the disease triangle factors, thereby achieving a significant lessening of the expected damages [68][69]. However, not all composts show this desirable property, and many studies have been performed to identify measurable parameters predicting it [70] and determine the procedures for obtaining it [71]. A number of studies demonstrated the crucial role of the leader microorganisms belonging to the complex of compost microbiota in giving high biological suppressive levels to the colonized organic matter that is eliminable by sterilization [72]. There may be a general or specific suppressiveness according to whether it is regulated by the totality of the members, or by a single or restricted group of the microbial community [73], respectively, which may be indicative of the spectrum of the target utilizations. Thus, specific suppressive or multi-suppressive substrates can be assembled. A recent study stated that the microbial diversity associated with the formation of very complex microbial structures during the composting process of green residues is critical for the specific suppression of soil-borne pathogens [74][75].
4.4. Wood Fibers
Wood fiber is characterized by a higher microbial activity than that of peat, similar to that observed in coir peat, but this is probably mainly due to N-immobilization processes, especially if not enriched by proper nitrogen sources [35][76][77]. However, there are a limited number of studies on wood fiber to assess its contribution to plant resistance against biotic and abiotic stress, unlike other wood and/or plant fiber by-products, such as sawdust, miscanthus, and reed [78][79]. Therefore, this can be considered a research field that is under continuous evolution and worthy of exploration.
5. Conclusions
Soilless culture provides solutions for resources-saving production systems, but continuous efforts are required to achieve highly sustainable production in circular-thought soilless cropping. Indeed, in such a transition to circular horticultural production, the transformation of green waste into shared resource plays a major role.
Their physicochemical characteristics may indeed give a relevant contribution to crop performance beyond the mere function of root support, which makes these materials a promising alternative to peat, as stand-alone or as single components for growing media. The presence of organic materials in the root zone of container-grown plants has been proved to play a relevant role in plant nutrition and irrigation. The action mechanisms of organic materials on plant nutrition are first an additional amount of nutrients available in the root zone for plants. Moreover, the chemical properties related to their ability of retaining and exchanging nutrient elements play an important role. Moreover, there is evidence of possible improved proliferation of organisms that may positively interact with plant nutrition and irrigation as in the case of mycorrhiza purification.
Different issues may arise from the use of alternative-to-peat organic materials, which include the possible presence of undesired organic compounds and excess of mineral elements, uncontrolled bioactivity in the root zone, the maintenance of substrate initial properties throughout the cultivation cycle, and the constant availability at market level of these materials. However, one of the main constraints to the use of organic growing substrates is probably represented by the high variability of these materials, which depends on both the feedstock used and the production process. Encouragingly, the application of standardized production procedures might partially overcome this problem.
This entry is adapted from the peer-reviewed paper 10.3390/agronomy11061236
References
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Proc. 2015, 3, 283–288.
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234.
- Majsztrik, J.C.; Ristvey, A.G.; Lea-Cox, J.D. Water and nutrient management in the production of container-grown ornamentals. Hortic. Rev. 2011, 38, 253–297.
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing water and nutrient losses from soilless cropping in southern Europe. Agric. Water Manag. 2020, 241, 106395.
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1–6.
- Bourguignon, D. Closing the Loop: New Circular Economy Package. Available online: (accessed on 10 April 2021).
- Schmilewski, G. Producing growing media responsibly to help sustain horticulture. Acta Hortic. 2014, 1034, 299–306.
- Raviv, M.; Lieth, J.H. Soilless Culture: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2008.
- Raviv, M. Composts in growing media: What’s new and what’s next? Acta Hortic. 2013, 982, 39–52.
- Bullock, C.H.; Collier, M.J.; Convery, F. Land use policy peatlands, their economic value and priorities for their future management—The example of Ireland. Land Use Policy 2012, 29, 921–928.
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: A life-cycle analysis. AMBIO J. Human Environ. 2005, 34, 456–461.
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18.
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82.
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of biochar application on nutritional quality of tomato (Lycopersicon esculentum). Crop. Pasture Sci. 2015, 66, 747–755.
- Suthar, R.G.; Wang, C.; Nunes, M.C.N.; Chen, J.; Sargent, S.A.; Bucklin, R.A.; Gao, B. Bamboo biochar pyrolyzed at low temperature improves tomato plant growth and fruit quality. Agriculture 2018, 8, 153.
- Sabatino, L.; Iapichino, G.; Mauro, R.P.; Consentino, B.B.; Pasquale, C.D. Poplar biochar as an alternative substrate for curly endive cultivated in a soilless system. Appl. Sci. 2020, 10, 1258.
- Carlile, W.R.; Raviv, M.; Prasad, M. Organic Soilless Media Components; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444636966.
- Bedussi, F.; Zaccheo, P.; Crippa, L. Pattern of pore water nutrients in planted and non-planted soilless substrates as affected by the addition of biochars from wood gasification. Biol. Fertil. Soils 2015, 51, 625–635.
- Jahromi, N.B.; Fulcher, A.; Walker, F.; Altland, J.; Wright, W.; Eash, N. Evaluating on-demand irrigation systems for container-grown woody plants grown in biochar-amended pine bark. HortScience 2018, 53, 1891–1896.
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assessment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J. Sci. Food Agric. 2017, 97, 3675–3684.
- Noguera, P.; Abad, M.; Puchades, R.; Maquieira, A.; Noguera, V. Influence of particle size on physical and chemical properties of coconut coir dust as container medium. Commun. Soil Sci. Plant Anal. 2003, 34, 593–605.
- Massa, D.; Malorgio, F.; Lazzereschi, S.; Carmassi, G.; Prisa, D.; Burchi, G. Evaluation of two green composts for peat substitution in geranium (Pelargonium zonale L.) cultivation: Effect on plant growth, quality, nutrition, and photosynthesis. Sci. Hortic. 2018, 228, 213–221.
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R.; Strik, B. Suitability of sphagnum moss, coir, and douglas fir bark as soilless substrates for container production of highbush blueberry. HortScience 2017, 52, 1692–1699.
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245.
- Mariotti, B.; Martini, S.; Raddi, S.; Tani, A.; Jacobs, D.F.; Oliet, J.A.; Maltoni, A. Coconut coir as a sustainable nursery growing media for seedling production of the ecologically diverse quercus species. Forests 2020, 11, 522.
- Lopez-Mondejar, R.; Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Canali, S.; Intrigiolo, F.; Pascual, J.A. Utilisation of citrus compost-based growing media amended with Trichoderma harzianum T-78 in Cucumis melo L. seedling production. Bioresour. Technol. 2010, 101, 3718–3723.
- Barker, A.V.; Bryson, G.M. Comparisons of composts with low or high nutrient status for growth of plants in containers. Commun. Soil Sci. Plant. Anal. 2006, 37, 1303–1319.
- Pane, C.; Celano, G.; Piccolo, A.; Villecco, D.; Spaccini, R.; Palese, A.M.; Zaccardelli, M. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem. Biol. Technol. Agric. 2015, 2, 1–13.
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Paull, R.E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 2012, 148, 138–146.
- Benito, M.; Masaguer, A.; Antonio, R.D.; Moliner, A. Use of pruning waste compost as a component in soilless growing media. Bioresour. Technol. 2005, 96, 597–603.
- Vandecasteele, B.; Boogaerts, C.; Vandaele, E. Combining woody biomass for combustion with green waste composting: Effect of removal of woody biomass on compost quality. Waste Manag. 2016, 58, 169–180.
- Hurley, S.; Shrestha, P.; Cording, A. Nutrient leaching from compost: Implications for bioretention and other green stormwater infrastructure. J. Sustain. Water Built Environ. 2017, 3, 04017006.
- Witcher, A.L.; Pickens, J.M.; Blythe, E.K. Container color and compost substrate affect root zone temperature and growth of “green Giant” arborvitae. Agronomy 2020, 10, 484.
- Graceson, A.; Hare, M.; Monaghan, J.; Hall, N. The water retention capabilities of growing media for green roofs. Ecol. Eng. 2013, 61, 328–334.
- Carlile, W.R.; Cattivello, C.; Zaccheo, P. Organic growing media: Constituents and properties. Vadose Zone J. 2015, 14, 1–13.
- Maher, M.; Prasad, M.; Raviv, M. Organic soilless media components. In Soilless Culture: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2008; pp. 459–504.
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrate for production of vegetable transplants: I. Physical properties of wood fiber substrates. Sci. Hortic. 2004, 100, 309–322.
- Boyer, C.R.; Gilliam, C.H.; Fain, G.B.; Gallagher, T.V.; Torbert, H.A.; Sibley, J.L. Production of woody nursery crops in clean chip residual substrate. J. Environ. Hortic. 2009, 27, 56–62.
- Jackson, B.E.; Wright, R.D.; Browder, J.F.; Harris, J.R.; Niemiera, A.X. Effect of fertilizer rate on growth of azalea and holly in pine bark and pine tree substrates. HortScience 2008, 43, 1561–1568.
- Frangi, P.; Amoroso, G.; Ferrini, F.; Fini, A. Growth of ornamental shrubs in wood fibre-based growing media. Acta Hortic. 2008, 801, 1571–1575.
- Handreck, K.; Black, N. Growing Media for Ornamental Plants and Turf, 3rd ed.; University of New South Wales Press: Sydney, NSW, Australia, 2002.
- Caron, J.; Elrick, D.E.; Beeson, R.; Boudreau, J. Defining Critical Capillary Rise Properties for Growing Media in Nurseries. Soil Sci. Soc. Am. J. 2005, 69, 794–806.
- Jahromi, N.B.; Walker, F.; Fulcher, A.; Altland, J.; Wright, W.C. Growth response, mineral nutrition, and water utilization of containergrown woody ornamentals grown in biochar-amended pine bark. HortScience 2018, 53, 347–353.
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298.
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrates for production of vegetable transplants II. The effect of wood fiber substrates and their volume weights on the growth of tomato transplants. Sci. Hortic. 2004, 100, 333–340.
- Farrell, C.; Cao, C.T.N.; Ang, X.Q.; Rayner, J.P. Use of water-retention additives to improve performance of green roof substrates. Acta Hortic. 2016, 1108, 271–278.
- Mulcahy, D.N.; Mulcahy, D.L.; Dietz, D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013, 88, 222–225.
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop. Sci. 2015, 201, 368–378.
- Awad, Y.M.; Lee, S.E.; Ahmed, M.B.M.; Vu, N.T.; Farooq, M.; Kim, I.S.; Kim, H.S.; Vithanage, M.; Usman, A.R.A.; Al-Wabel, M.; et al. Biochar, a potential hydroponic growth substrate, enhances the nutritional status and growth of leafy vegetables. J. Clean. Prod. 2017, 156, 581–588.
- Di Lonardo, S.; Baronti, S.; Vaccari, F.P.; Albanese, L.; Battista, P.; Miglietta, F.; Bacci, L. Biochar-based nursery substrates: The effect of peat substitution on reduced salinity. Urban For. Urban Green. 2017, 23, 27–34.
- Evans, M.R.; Jackson, B.E.; Popp, M.; Sadaka, S. Chemical properties of biochar materials manufactured from agricultural products common to the southeast united states. Horttechnology 2017, 27, 16–23.
- Massa, D.; Bonetti, A.; Cacini, S.; Faraloni, C.; Prisa, D.; Tuccio, L.; Petruccelli, R. Soilless tomato grown under nutritional stress increases green biomass but not yield or quality in presence of biochar as growing medium. Hortic. Environ. Biotechnol. 2019, 60, 871–881.
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–12.
- Zulfiqar, F.; Allaire, S.E.; Akram, N.A.; Méndez, A.; Younis, A.; Peerzada, A.M.; Shaukat, N.; Wright, S.R. Challenges in organic component selection and biochar as an opportunity in potting substrates: A review. J. Plant Nutr. 2019, 42, 1386–1401.
- San Bautista, A.; Rueda, R.; Pascual, B.; Maroto, J.V.; López-Galarza, S. Influence of different substrates and nutrient solutions on the yields and the incidence of abiotic disorders of broccoli. Acta Hortic. 2005, 697, 275–280.
- Hongpakdee, P.; Ruamrungsri, S. Water use efficiency, nutrient leaching, and growth in potted marigolds affected by coconut coir dust amended in substrate media. Hortic. Environ. Biotechnol. 2015, 56, 27–35.
- Olle, M.; Ngouajio, M.; Siomos, A. Vegetable quality and productivity as influenced by growing medium: A review. Zemdirbyste 2012, 99, 399–408.
- Massa, D.; Prisa, D.; Lazzereschi, S.; Cacini, S.; Burchi, G. Heterogeneous response of two bedding plants to peat substitution by two green composts. Hort. Sci. 2018, 45, 164–172.
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306.
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant. Sci. 2019, 10, 1–17.
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant. Signal. Behav. 2010, 5, 635–643.
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27.
- Ros, M.; Hernandez, M.T.; Garcia, C.; Bernal, A.; Pascual, J.A. Biopesticide effect of green compost against Fusarium wilt on melon plants. J. Appl. Microbiol. 2005, 98, 845–854.
- Yogev, A.; Raviv, M.; Hadar, Y.; Cohen, R.; Katan, J. Plant waste-based composts suppressive to diseases caused by pathogenic Fusarium oxysporum. Eur. J. Plant. Pathol. 2006, 116, 267–278.
- De Corato, U.; Patruno, L.; Avella, N.; Lacolla, G.; Cucci, G. Composts from green sources show an increased suppressiveness to soilborne plant pathogenic fungi: Relationships between physicochemical properties, disease suppression, and the microbiome. Crop. Prot. 2019, 124, 104870.
- Stewart-Wade, S.M. Efficacy of organic amendments used in containerized plant production: Part 1—Compost-based amendments. Sci. Hortic. 2020, 266, 108856.
- St. Martin, C.C.G.; Brathwaite, R.A.I. Compost and compost tea: Principles and prospects as substrates and soil-borne disease management strategies in soil-less vegetable production. Biol. Agric. Hortic. 2012, 28, 1–33.
- Pane, C.; Piccolo, A.; Spaccini, R.; Celano, G.; Villecco, D.; Zaccardelli, M. Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl. Soil Ecol. 2013, 65, 43–51.
- Pane, C.; Chiantese, C.; Scala, F.; Bonanomi, G. Assessment of gardening growing media suppressiveness against Rhizoctonia damping-off disease. J. Plant. Pathol. 2013, 95, 401–405.
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144.
- Pane, C.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Disease suppressiveness of agricultural greenwaste composts as related to chemical and bio-based properties shaped by different on-farm composting methods. Biol. Control. 2019, 137, 104026.
- Malandraki, I.; Tjamos, S.E.; Pantelides, I.S.; Paplomatas, E.J. Thermal inactivation of compost suppressiveness implicates possible biological factors in disease management. Biol. Control. 2008, 44, 180–187.
- Hoitink, H.A.J.; Boehm, M.J. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446.
- Pane, C.; Sorrentino, R.; Scotti, R.; Molisso, M.; Di Matteo, A.; Celano, G.; Zaccardelli, M. Alpha and beta-diversity of microbial communities associated to plant disease suppressive functions of on-farm green composts. Agriculture 2020, 10, 113.
- Scotti, R.; Mitchell, A.L.; Pane, C.; Finn, R.D.; Zaccardelli, M. Microbiota characterization of agricultural green waste-based suppressive composts using omics and classic approaches. Agriculture 2020, 10, 61.
- Gruda, N. Sustainable peat alternative growing media. Acta Hortic. 2012, 927, 973–980.
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Prod. 2020, 256, 120323.
- Ehret, D.L.; Helmer, T. A new wood fibre substrate for hydroponie tomato and pepper crops. Can. J. Plant. Sci. 2009, 89, 1127–1132.
- Vandecasteele, B.; Muylle, H.; De Windt, I.; Van Acker, J.; Ameloot, N.; Moreaux, K.; Coucke, P.; Debode, J. Plant fibers for renewable growing media: Potential of defibration, acidification or inoculation with biocontrol fungi to reduce the N drawdown and plant pathogens. J. Clean. Prod. 2018, 203, 1143–1154.
