In 2014, CRSPR-Cas9 was used to create the first needle-leaf mutant in tomato, by knocking out
Argonaute 7 [
33]. Many studies have since been published on its possible applications in protecting plants against biotic and abiotic stresses, and improving fruit quality, plant architecture, and shelf life [
34]. Currently, the system is in the research stage for many fruits and vegetables crops, such as cabbage, mustard, tomato, and watermelon.
Most gene-editing studies have evaluated mutation efficiency in terms of the number of albino plants obtained after mutation of the endogenous phytoene desaturase (
PDS) gene. The disruption of
PDS impairs the production of chlorophyll and carotenoid, generating an easily identifiable albinism phenotype in plants. However, the products of gene editing obtained in this way have no economic value [
35,
36,
37]. Because of its high economic value and the availability of Agrobacterium-mediated transformation, tomato has become a model crop for testing CRISPR-Cas9 applications (
Figure 2).
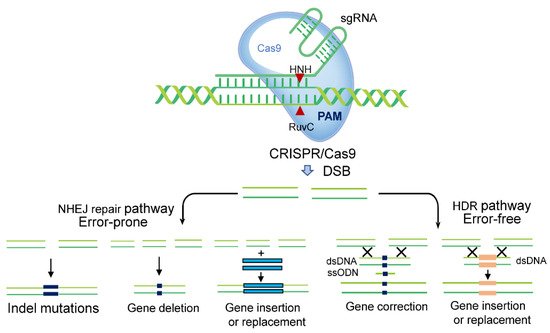
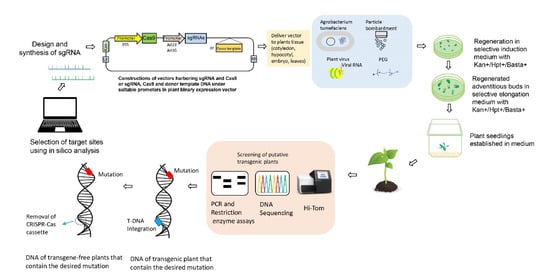
Figure 2. CRISPR-Cas9 mediated genome editing. (I) Selection of the desired genomic DNA target, and recognition of protospacer adjacent motif (PAM) sequences before 20 bp sequences. Design of the sgRNA using online bioinformatics tools. (II) Cloning of designed sgRNAs, and binary vector construction using promoters. (III) The delivery of CRISPR-Cas editing reagents into plant cells. The vector can be transferred into the plant via Agrobacterium tumefaciens, nanoparticles, biolistic bombardment, or polyethylene glycol (PEG). Alternatively, plant RNA viruses have been used to induce heritable genome editing. When the cassette harbouring the sgRNA, RNA mobile element, and tobacco rattle virus (TRV) is transformed into the Cas9 expressing plants, the systemic spread of sgRNA will introduce heritable genome editing. (IV) Plant transformation and development of transgenic plants. (V) Genotyping of transgenic plants. (VI) Transgene-free plants with the desired mutation are obtained.
2.1. Improvement of Biotic Stress Resistance
Two strategies have been used to improve plant resistance to viruses: (1) designing sgRNAs and targeting the virus genome; or (2), modifying the fruit crop genes in the antiviral pathway. The binding of virus genome linked protein (VPg) to the plant protein ‘eukaryotic translation initiation factor 4E’ (eIF4E) is key in Y virus infection of plants. Mutation of a key site of eIF4E can affect the virus–plant interaction, and mediate plant resistance to this virus [
38]. In cucumbers, using CRISPR-Cas to target the N′ and C′ ends of eIF4E-produced nontransgenic homozygous plants in the T3 generation; these showed immunity to cucumber vein yellow virus and pumpkin mosaic virus, and resistance to papaya ring spot mosaic virus (PRSV-W) [
39].
CRISPR-Cas9 can generate mutations in the coding and noncoding regions of geminivirus, effectively reducing its pathogenicity. In
Nicotiana benthamiana, sgRNA-Cas9 constructs target beet severe curly top virus (a geminivirus), inhibiting its accumulation in leaves [
40]. Geminivirus noncoding-region mutations are believed to reduce or even inhibit its replication ability. Compared with coding-region mutations, noncoding-region mutations generate fewer viral variants [
41].
Fungi cause many diseases, potentially causing severe losses in crop yield and quality. For instance, downy and powdery mildews cause serious economic losses in tomato [
42].
Arabidopsis thaliana DMR6 (down mildew resistant) is a member of the 2-oxoglutarate oxygenase Fe(II)-dependent superfamily and is involved in salicylic acid homeostasis. Overexpression of
DMR6 in plants can reduce susceptibility to downy mildew [
43]. The
DMR6 mutation obtained using CRISPR-Cas9 to knock out the homologous genes in tomato showed resistance to
Pseudomonas syringae,
Phytophthora and
Xanthomonas spp. [
44].
Mlo1 (Mildew resistant locus 1) encodes a membrane-associated protein and is a powdery mildew disease-sensitivity gene. In tomato,
Mlo1 mutants obtained via gene editing exhibited resistance to the powdery mildew
Oidium neolycopersici. Further, a mutant free of mlo1 T-DNA was obtained by selfing T0 generation plants [
45].
The fungal pathogen
Fusarium oxysporum can cause
Fusarium wilt disease in fruit and vegetable crops [
46]. In tomatoes,
Solyc08g075770-knockout via CRISPR-Cas9 resulted in sensitivity to
Fusarium wilt disease [
47]. In watermelons, the knockout of
Clpsk1, encoding the Phytosulfokine (PSK) precursor, confers enhanced resistance to
Fusarium oxysporum f.sp.
niveum (FON) [
48].
Botrytis cinerea, an airborne plant pathogen that infects fruit and vegetable crops, causes great economic losses. Its initial symptoms are not obvious, and the lack of effective pesticides makes its prevention and control difficult. Pathogens can be effectively controlled in crops by the use of genetic resources that convey heritable resistance. In tomatoes, mutations in
MAPK3 (mitogen-activated protein kinase 3) produced using CRISPR-Cas9 induce resistance to
Botrytis cinerea [
49].
The bacterial pathogen
Pseudomonas syringae causes leaf spot diseases in crops, severely impacting the yield and sensory qualities of fruits and vegetables. In
Arabidopsis thaliana, CRISPR-Cas9 was used to mutate the C-terminal jasmonate domain (JAZ2Δjas) of
JAZ2 (jasmonate ZIM domain protein 2), causing expression of JAZ2 repressors; these repressors confer resistance to
Pseudomonas syringae [
50].
2.2. Abiotic Stress Resistance Improvement
With climate change, crop production is exposed to increased potential risks of abiotic stress. Although traditional breeding can to some extent ensure stable crop production, the application of new technologies to rapidly obtain new crop germplasm resources capable of responding to abiotic stress is essential for accelerating the cultivation of new varieties [
51]. The emergence of CRISPR-Cas9 gene editing has shortened the time required to create new varieties. Brassinazole-resistant 1 gene (
BZR1) participates in various brassinosteroid (BR) mediated development processes. The CRISPR mediated mutation in
BZR1 impaired the induction of
RESPIRATORY BURST OXIDASE HOMOLOG1(
RBOH1) and the production of H
2O
2. Exogenous H
2O
2 recovered the heat tolerance in tomato bzr1 mutant [
52]. Further, new cold- and drought-tolerant germplasms can be created using gene-editing, for instance, of
CBF1 (C-repeat binding factor 1), which regulates cold tolerance in plants, and
MAPK3, which participates in the drought stress response to protect plant cell membranes from peroxidative damage in tomatoes [
53,
54].
2.3. Herbicide Resistance Improvement
Weeds are an important cause of stress that affect vegetable yield and quality, and selective herbicides are often used to control weed growth during cultivation. To obtain herbicide-resistant fruits and vegetables for field production, CRISPR-Cas9 gene editing was used for site-directed mutagenesis of the herbicide target gene acetolactate synthase (ALS) in watermelon, yielding a herbicide-resistant watermelon germplasm [
55]. Cytidine base editing (CBE) was used for cytidine editing of key
ALS sites in tomato and potato, resulting in amino acid mutations. Up to 71% of edited tomato plants exhibited resistance to the pesticide chlorsulfuron, and of the edited tomato and potato plants, 12% and 10%, respectively, were free of GM components [
56].
Phelipanche aegyptiaca, an obligate weedy plant parasite, requires the host roots to release the plant hormone strigolactone (SL) to promote seed germination; CRISPR-Cas9 was used to mutate carotenoid dioxygenase 8 (
CCD8), a key enzyme in the carotenoid synthesis pathway that produces SLs in tomato, and More Axillary Growth1 (
MAX1), which is involved in the synthesis of SLs, thereby significantly reducing SL content, and creating
P. aegyptiaca-resistant tomato plants [
57,
58].
2.4. Fruit and Vegetable Quality Improvement
The primary goal in fruit and vegetable breeding is to improve quality and prolong shelf life after harvest. Quality refers to both external and internal factors. External quality refers to fruit size, colour, and texture, which can be discerned by the naked eye. Internal quality must be measured using equipment, and includes the levels of nutrients such as sugars, vitamins, and bioactive compounds including lycopene, anthocyanins, and malate. For example, in tomato, the ovary locule number, which determines 50% of the genetic variation in fruit size, is determined by multiple QTLs [
59]. Researchers at Cold Spring Harbor Laboratory designed eight sgRNAs and used CRISPR-Cas9 to edit the promoter region of the tomato CLAVATA-WUSCHEL (CLV-WUS) stem cell gene
CLV3 to obtain fruits that are larger and more numerous than wild-type fruits [
60]; editing of fruit-size determining QTLs, such as the QTLs for locule number (lc) and fasciated number (fas), generated germplasm resources with an increased number of locules [
61].
Fruit and vegetable colour and texture are important traits for consumers. For example, European and American consumers prefer red tomatoes, whereas Asian consumers prefer pink tomatoes [
62,
63]. CRISPR-Cas was used to modify phytoene synthase 1 (
PSY1), MYB transcription factor 12 (
MYB12), and anthocyanin 2 (
ANT2) to obtain yellow, pink, and purple tomatoes, respectively [
64,
65,
66]. The carotenoid isomerase gene of Chinese kale (
BoaCRTISO) is responsible for catalysis, then conversion of lycopene precursors to lycopene. When
BoaCRTISO was targeted and edited, the colour of mutants changed from green to yellow [
67]. The primary goal of improving the intrinsic quality of fruits and vegetables is to improve their nutrient and bioactive compound content. Carbohydrates and vitamins are essential nutrients. Many genes are involved in the synthesis and metabolism of sucrose and carotenoids. One of the carotenoids, provitamin A, can be absorbed by the human body and converted into vitamin A. For example, CRISPR-Cas was used to knock out
MPK20 (mitogen-activated protein kinase 20), blocking the transcription and protein products of multiple genes in the sucrose metabolism pathway [
68]. Biofortification, the biotechnological improvement of the absorption, transport, and metabolism of minerals by plants, increases the levels of micronutrients that are beneficial to human health; long-term consumption of these micronutrients can effectively prevent cardiovascular disease and cancer [
69].
Anthocyanins [
70], malate [
71], γ-aminobutyric acid (GABA) [
72], and lycopene [
73] are bioactive compounds. Adjusting key metabolic-pathway-related genes via CRISPR-Cas9 can enrich these nutrients in fruits. For example, in tomatoes, butylamine content was increased 19-fold through editing multiple genes in the GABA synthesis pathway, and malate content was improved by regulating aluminium-activated malate transporter (
ALMT9) [
72].
CRISPR-Cas9 can also be used to reduce the content of substances in vegetables that are not conducive to human health, by targeting mutations that inactivate genes in biosynthetic pathways. In potato tubers, for example, excessive content of steroidal glycoalkaloids (SGAs), such as α-solanine and α-chaconine, affects their taste and makes them less safe for human consumption, hence low content is an indicator of high quality. CRISPR-Cas9 was used to delete
St16DOX (steroid 16α-hydroxylase) in the potato SGA biosynthetic pathway, resulting in SGA-free potato lines [
74].
Prolonged shelf-life is an important breeding goal in fruit and vegetable production. CRISPR was used to knock out ripening inhibitor (
RIN) or DNA demethylase (DNA demethylase 2,
DML2) to slow fruit ripening, thereby prolonging their shelf life. However, regulating these two genes in fruit alters peel colour and reduces flavour and nutritional value, severely reducing the fruit’s palatability and sensory qualities [
75,
76]. In tomatoes, inhibiting the expression of the pectate lyase (
PL) and alcobaca (
ALC) genes effectively extended shelf life, without affecting the sensory qualities or nutritional value [
77,
78].