3.1. Genetic Regulation of FliC and FliD
The genetic organization of the flagellar components is highly complex and relatively well conserved across bacteria with the same type of flagellar arrangement (i.e., peritrhichous, monotrichous/polar, etc.) but quite different between them [
1,
99,
100]. The bacterial flagellar regulon commonly consists of dozens of genes grouped in several operons, encoding all structural proteins of the flagellum, the chemosensory apparatus and the regulators that control gene expression. For example, there are about 50 genes involved in the synthesis of the polar
P. aeruginosa flagella, distributed in 17 putative operons comprising 41 flagellar genes (26 encoding structural proteins, eight encoding regulators and six involved in the export apparatus), linked to nine genes involved in chemotaxis and clustered in three regions of the chromosome constituting the
fla regulon [
101,
102]. On the other hand, in peritrichous bacteria such as
Salmonella spp. and
Escherichia coli, the flagellar regulon contains 70 genes distributed in at least 25 operons [
79,
99]. The transcription of these genes is hierarchical and controlled by different promoter classes that are temporally regulated (
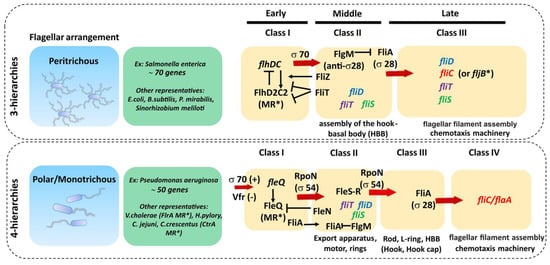
Figure 4,
Table 1). In peritrichous bacteria, flagellar genes are organized into three classes (I-III), starting with the transcription of the class I master operon as a response to the environmental stimuli that leads to the synthesis of the transcriptional regulator FlhDC. This regulator, in turn, activates the transcription of the class II genes responsible for the assembly of the hook-basal body (HBB) complex. Among class II genes are two crucial regulators of the class III genes: transcription factor FliA (σ
28) responsible for the transcription of class III genes, and FlgM, which acts as a FliA inhibitor. Once the HBB is assembled, FlgM is exported out of the cell via the HBB central channel, releasing FliA to interact with RNA polymerase and guide the transcription of the class III genes, including flagellin. This three-tiered organization ensures that cells produce flagella in response to the environmental signals and that energetically costly synthesis of the filament proceeds only when enough HBBs have been formed. An exception is the
Actinoplanes missouriensis zoospore in which there is no hierarchical coordination and 33 flagellar genes are transcribed simultaneously during the sporangium formation [
103]. The filament cap gene
fliD belongs to the group of class II genes, whereas flagellin genes belong to class III. Although
fliD is expressed as a class II gene, it is not assembled into the growing flagellar structure until the hook and the junction proteins are expressed from class III (including flagellin genes) and incorporated into the flagellar organelle [
104]. Hence, this cascade serves to control the timing of gene expression to coincide with the assembly of the flagellar apparatus and filament.
Figure 4. Genetic organization of main flagellar proteins involved in filament formation. The transcription of flagellar genes is hierarchical and controlled by different promoter classes that are temporally regulated. In peritrichous bacteria, flagellar genes are organized into three classes (I–III) and in monotrichous/polar bacteria the flagellar genes are organized into four classes, both starting with the transcription of the class I master regulator (MR*). Red arrows indicate the proteins and/or transcription factors responsible for the transcription activation of each corresponding class of flagellar genes. Black narrow arrows indicate positive activation/induction and black arrows with a vertical line tip (|) indicate inhibition. Genes involved in filament synthesis and their cognate chaperones are represented in italic colored font.
Table 1. Flagellins and FliD and their regulators.
| Protein |
Function |
Sigma Factor |
Class in Flagellar Regulatory Hierarchy |
Transcriptional Expression Regulators |
Post-Transcriptional Regulators |
Specific Secretion Chaperone |
| 3-Tiered |
4-Tiered |
| Flagellin (FliC, FlaA) |
Structural component of the filament |
Sigma28
Sigma54
Sigma43 |
Class III gene |
Class IV gene |
CodY
Environmental factors (nutrients, c-di-GMP, ppGpp, BCAA, temperature…) |
Self-regulating
CsrA-FliW |
FliS |
| FliD |
Filament cap |
Sigma70
Sigma28
Sigma54 |
Class II and III |
Class II |
Cognate flagellin |
? |
FliT |
As mentioned above,
S. Typhimurium exhibits phase variation, or alternate expression of two flagellin genes,
fliC and
fljB. This process is regulated by a control region upstream of
fljB that is a subject of inversion, which orients the region forward and generates a promoter. This allows co-transcription of
fljB and
fljA, a transcriptional repressor that inhibits the expression of FliC [
105].
Recently, Rao et al. found that flagellar gene expression in
Salmonella is bimodal, meaning that in a population of genetically identical cells under the same conditions only a fraction of them is motile [
106,
107]. This bimodality is present at both the class II and class III levels and is governed by separate mechanisms. While a double negative feedback loop involving two flagellar regulatory proteins, RflP and FliZ, controls the expression of the class II genes in response to nutrient availability, class III gene expression is tuned by the secretion of FlgM and there is a minimum number of HBBs necessary for the cell to pass this checkpoint.
In bacteria with a polar flagellum, flagellar genes are transcribed in a four-tiered hierarchy, with genes encoding components of the HBB split between class II and class III (
Figure 4) [
101]. Gene regulation in
Pseudomonas is more complex and involves the activation of the two-component system FleS-FleR and another sigma factor RpoN (σ
54) to activate class III genes. In addition, unlike in
Salmonella, FliA (σ
28) is constitutively expressed in
Pseudomonas independently of other flagellar genes, which makes it one of the class I genes [
101].
In bacteria with multiple flagellins, such as
Helicobacter and
Campylobacter, expression is under the control of different sigma factors with FlaA under control of σ
28, while FlaB is under control of σ
54 [
108,
109]. Although the major flagellin is usually under the control of σ
28, there are exceptions such as
V. cholera and
S. oneidensis in which the major flagellin is dependent on σ
54, while commensal gut bacteria of
Eubacterium and
Roseburia species are under the control of σ
28 and σ
43 [
110,
111]. In the phylogenetically related
Butyrivibrio fibrisolvens, transcription of one
fliC gene is driven from two different promoters, yielding two transcripts with alternative transcription start-sites [
111].
In some Gram-positive bacteria, changes in the environmental conditions such as nutrient limitation induce variations in the levels of the intracellular messengers guanosine tetra/pentaphosphate, (p)ppGpp, guanosine nucleoside triphosphate (GTP) and branched chain amino-acid pools. These variations are detected by a conserved GTP-sensing protein CodY and a global regulator CsrA that modulate flagellin expression. Cell motility can be repressed under elevated intracellular cyclic-di-GMP levels, by impeding transcription of some of the flagellum genes [
112,
113,
114,
115,
116]. Indeed, the expression of
fliD and
fliC is repressed in phosphodiesterase 3 (PDE3) knockout mutants triggered by elevated c-di-GMP accumulation [
117].
Because the presence of flagellin can be deleterious to the bacterium on account of inducing host immunity, flagellated bacteria downregulate (or turn off) flagellin expression during the host invasion to avoid host immune responses. Therefore, normal microbiota within the healthy adult mammalian gut has been shown to have overall relatively low levels of flagellin expression, while TLR5−/− mice exhibited a diversity of gut microbiome members with overexpressed flagellar genes [
118,
119].
Both commensal gut strains of motile
E. coli and pathogenic
S. Typhimurium strains strongly down-regulate their genes coding for flagellar machinery and lose their motility once inside the host [
120,
121]. Under environmental temperature conditions (22–30 °C), the expression of flagellar genes in the human pathogens
Listeria monocytogenes and
Legionela pneumophila is normal, but significantly reduced when raised to 37 °C, revealing temperature-dependent transcription mainly controlled by the protein GmaR, which acts as a protein expression thermostat [
122,
123]. These types of system provide a pathogen with the ability to turn off immune-stimulating antigens before they trigger adverse host defense mechanisms once inside their target host.
Regulation of flagellin expression also occurs on the post-transcriptional level by proteins that bind to the untranslated leader region of the flagellin mRNA, affecting transcript stability and/or ribosome access [
124,
125,
126]. For instance, the RNA-binding protein CsrA of
B. burgdorferi specifically mediates synthesis of the major flagellin by inhibiting translation initiation of its transcript. In some cases, post-transcriptional regulators repress the accumulation of flagellin when cells are defective for flagellar hook assembly [
127,
128].
An assembly checkpoint that prevents flagellin translation and assembly prior to hook completion was discovered in
B. subtilis. This is governed by a homeostatic mechanism in which the flagellin protein itself is a critical regulator. In a partner switching mechanism, the flagellar assembly factor FliW binds flagellin, while a global regulator CsrA binds flagellin mRNA, repressing its translation. After completion of the hook, flagellin is secreted, and the released FliW binds CsrA, thus derepressing flagellin translation [
129]. An interesting case is
A. missouriensis, in which all flagellar genes are transcribed simultaneously during sporangium formation and there is no checkpoint mechanism in the process of flagellar gene transcription to optimize the efficiency of the flagellar assembly [
103]. The post-transcriptional level of regulation between protein production and assembly could play an important role, although this remains unknown.