Allergic diseases are highly prevalent disorders, mainly in industrialized countries where they constitute a high global health problem. Allergy is defined as an immune response “shifted toward a type 2 inflammation” induced by the interaction between the antigen (allergen) and IgE antibodies bound to mast cells and basophils that induce the release of inflammatory mediators that cause the clinical symptoms. Currently, allergen-specific immunotherapy (AIT) is the only treatment able to change the course of these diseases, modifying the type 2 inflammatory response by an allergenic tolerance, where the implication of T regulatory (Treg) cells is considered essential.
- T-cell epitopes
- synthetic peptides
- peptide-allergen immunotherapy
- T regulatory response
- IL-35
- IL-10
- vaccines
- olive pollen allergy
- Ole e 1
1. Introduction of Allergen-Specific Immunotherapy (AIT)
AIT is considered a therapeutic vaccine that establishes tolerance against specific allergens[1] . This response mainly consists in the production of allergen-specific IgG antibodies that can block IgE binding to allergens, and alterations in the cellular immune response, particularly a reduction of allergen-specific Th2 responses [2]. Thus, tolerance induction is dependent on the immunogenicity and allergenicity of the allergen used as a vaccine. In fact, although the current AIT guidelines are effective, in some patients AIT may cause side effects that other formulations as modified proteins or peptides could avoid.
2. Peptide Allergen Immunotherapy: State of the Art
In the era of molecular diagnosis and treatments, new AIT approaches are being developed to decrease the allergenicity and adverse effects, as well as shorten the duration of the convectional AIT [3][4]. To achieve this, studies have been conducted on the use of hypoallergens, recombinant proteins, and methods other than subcutaneous and sublingual administration are under studying [5]. One of the most promising approaches is the use of peptides derived from the main allergens [6].
This new therapy uses soluble synthesized allergen fragments of variable lengths and is based on the primary structure of the allergen. Depending on the length of the fragments and their ability to induce tolerance, peptide-based vaccines can be divided into those that use IgE-mediated peptides and other using T-cell peptides [7].
Vaccines designed using IgE-mediated peptides consist of long peptides (20–40 amino acids) that share main B-cell epitopes of the allergens; therefore, these peptides depend on the folded tertiary structure. On the other hand, the use of short synthetic peptides (10–17 amino acids), called T-cell peptides, is based on the lack of conformational B-cell epitopes. In theory, after peptide processing, the immune response should be led by Th1 and Treg responses, with a secretion of IL-10 that decreases eosinophils, basophils and mast-cells recruitment to the affected tissues and weakens the ability to release mediators. Moreover, this type of peptides is unable to bind to IgE-FcεRI on effector cells, mainly due to the small peptide size [6][8]
AIT with peptides has been studied for more than 20 years, and the first clinical trials were developed against cat allergy[9][10][11][12][13][14][15][16][17][18], house-dust-mites allergy (HDM) [19][20][21][22], and pollen allergies [23][24][25][26], but different effective clinical results have been obtained [27]. Regarding HDM, different types of peptides from Der p 1, one of the major allergens of Dermatophagoides pteronyssinus, have been analysed [28][29][30]. One of the most recent study that failed to achieve a clinically significant benefit for the treatment against cat allergies was a large-scale phase III study using a mix of peptides from Fel d 1 (major cat allergen) containing T-cell epitopes The authors concluded that the mechanisms of IT with peptide allergens could be different from the whole-allergen IT and suggested that the decrease of CRTh2 could result in a failure to recruit and activate these cells, thereby reducing Th2 inflammatory responses in the airways [31].
3. Olive Pollen Allergy
Olive (Olea europaea) pollen is one of the most important causes of respiratory allergy in the Mediterranean area and some regions of North America, South Africa, Japan, and Australia [30] Specifically in Spain, it is the second most common cause of respiratory allergy after pollinosis to grass (approximately a 60% of all pollen-allergic patients are sensitized to olive pollen). Moreover, in certain areas of Andalusia (south of Spain), it is the most common respiratory allergy, with 84% of pollen-allergic subjects sensitized to olive pollen. In addition, the number of olive trees around the world is expected to increase considerably due to the health benefits of the Mediterranean diet, of which olive oil is a staple, thereby raising concerns that the number of patients with this pollinosis will increase worldwide [31].
At least 20 proteins with allergenic activity have been found in the olive pollen, and Ole e 1 is considered the major allergen. It is a 145-aa glycoprotein with sequence microheterogeneity, highly dependent on the olive cultivar analyzed [32][33]. The importance of this allergen also resides in the high degree of sequence homology with other main allergens from theOleaceaefamily as Fra e 1 (Fraxinum) and Syr v 1 (Syringa), which are responsible of a substantial percentage of pollinosis in Central Europe [33][34]. Besides Ole e 1, a total of fifteen olive allergens (Ole e 1 to 15) have been characterized [35][36][37], several of which (e.g., Ole e 10, Ole e 7) are minority proteins in the whole-pollen extract though are major allergens in regions with extremely high antigenic load and are associated with the most severe symptoms of the disease (severe asthma) [38][39].
Currently, the standard treatment for olive-pollen-allergic patients is based on specific immunotherapy with whole-olive-pollen extracts. Despite improvements in the extracts used in AIT, the effectiveness of this strategy is highly variable, as it depends on the patient’s own sensitization, the severity of the clinical manifestations, and the treatment itself (the difficulty of standardizing allergenic extracts causes great variability in their therapeutic potential).
Besides, with AIT there is a risk that allergic patients may become sensitized to other components present in the extracts, an aspect of special relevance in olive pollinosis since minor components of the pollen (e.g., Ole e 10, Ole e 7) can be especially allergenic and could induce severe clinical symptoms in high doses. Consequently, the search for improvements to these types of vaccines is one of the most pressing objectives of research in the field of allergy. The design of new vaccines with allergen-derived peptides could offer multiple advantages as discussed above.
4. Ole e 1-Peptides
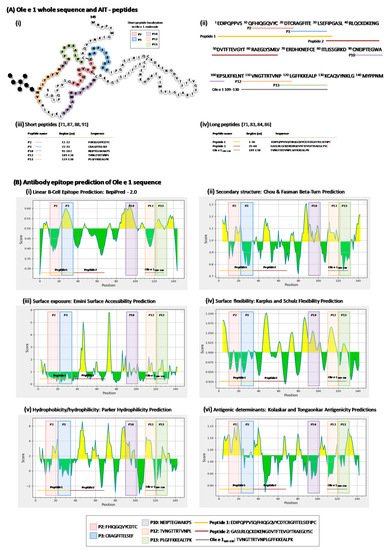
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13071007
References
- Passalacqua, G.; Bagnasco, D.; Ferrando, M.; Heffler, E.; Puggioni, F.; Canonica, G.W. Current insights in allergen immunotherapy. Ann. Allergy Asthma. Immunol. 2018, 120, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Eckl-Dorna, J.; Villazala-Merino, S.; Linhart, B.; Karaulov, A.V.; Zhernov, Y.; Khaitov, M.; Niederberger-Leppin, V.; Valenta, R. Allergen-specific antibodies regulate secondary allergen-specific immune responses. Front. Immunol. 2019, 9, 3131. [Google Scholar] [CrossRef]
- Komlósi, Z.I.; Kovács, N.; Sokolowska, M.; van de Veen, W.; Akdis, M.; Akdis, C.A. Highlights of Novel Vaccination Strategies in Allergen Immunotherapy. Immunol. Allergy Clin. N. Am. 2020, 40, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A. Perspectives in Allergen-Specific Immunotherapy: Molecular Evolution of Peptide- and Protein-Based Strategies. Curr. Protein. Pept. Sci. 2020, 21, 203–223. [Google Scholar] [CrossRef]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, present, and future of allergen immunotherapy vaccines. Allergy 2021, 76, 131–149. [Google Scholar] [CrossRef]
- Calzada, D.; Baos, S.; Cremades, L.; Cárdaba, B. New Treatments for Allergy: Advances in Peptide Immunotherapy. Curr. Med. Chem. 2018, 25, 2215–2232. [Google Scholar] [CrossRef]
- Marth, K.; Focke-Tejkl, M.; Lupinek, C.; Valenta, R.; Niederberger, V. Allergen Peptides, Recombinant Allergens and Hypoallergens for Allergen-Specific Immunotherapy. Curr. Treat. Options Allergy 2014, 26, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Montagni, M.; Ridolo, E. The efficiency of peptide immunotherapy for respiratory allergy. Expert Rev. Clin. Pharmacol. 2016, 9, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Ying, S.; Kay, A.B.; Larché, M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin. Exp. Allergy 2005, 35, 52–58. [Google Scholar] [CrossRef]
- Alexander, C.; Tarzi, M.; Larché, M.; Kay, A.B. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy 2005, 60, 1269–1274. [Google Scholar] [CrossRef]
- Verhoef, A.; Alexander, C.; Kay, A.B.; Larché, M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005, 2, e78. [Google Scholar] [CrossRef]
- Worm, M.; Lee, H.H.; Kleine-Tebbe, J.; Hafner, R.P.; Laidler, P.; Healey, D.; Buhot, C.; Verhoef, A.; Maillère, B.; Kay, A.B.; et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J. Allergy Clin. Immunol. 2011, 127, 89–97. [Google Scholar] [CrossRef]
- Larché, M.; Patel, D.; Patel, P.; Salapatek, A.M.; Laidler, P.; Hafner, R. Safety and efficacy of Fel d 1 derived peptide immunotherapy in a double-blind, placebo-controlled environmental exposure chamber (EEC) study. Allergy 2012, 67, 1–97. [Google Scholar]
- Hafner, R.P.; Patel, P.; Salapatek, A.M.; Laider, P.; Larché, M.; Patel, D. Fel d 1 peptide antigen desensitization safety and efficacy in a double-blind, placebo-controlled environmental exposure chamber study. World Allergy Organ. J. 2013, 6 (Suppl. S1), P150. [Google Scholar] [CrossRef]
- Patel, D.; Couroux, P.; Hickey, P.; Salapatek, A.M.; Laider, P.; Larché, M.; Hafner, R.P. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: A randomized, placebo-controlled study. J. Allergy Clin. Immunol. 2013, 131, 103–109. [Google Scholar] [CrossRef]
- Hafner, R.P.; Couroux, P.; Armstrong, K.; Patel, D.; Larché, M. Two Year Persistent Treatment Effect Achieved After 4 Doses of Cat-Peptide Antigen Desensitization (Cat- PAD) in an Environmental Exposure Chamber (EEC) Model of Cat Allergy. J. Allergy Clin. Immunol. 2013, 131, AB147. [Google Scholar] [CrossRef]
- Hafner, R.P.; Couroux, P.; Armstrong, K.; Patel, D.; Larché, M.; Haumann, B. Total Nasal Symptom Scores are reduced in an EEC model of cat allergy two years after administration of 4 doses of Cat-PAD. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 647. [Google Scholar]
- Pfaar, O.; Klimek, L.; Varga, E.M. Fel d 1 synthetic peptides (Cat-PAD)—Good news for cat owners with children? Pediatr. Allergy Immunol. 2016, 27, 666–670. [Google Scholar] [CrossRef]
- Kündig, T.M.; Senti, G.; Schnetzler, G.; Wolf, C.; Vavricka, B.M.P.; Fulurija, A.; Hennecke, F.; Sladko, K.; Jennings, G.T.; Bachmann, M.F. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J. Allergy Clin. Immunol. 2006, 117, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Larché, M.; Hickey, P.; Hebert, J.; Hafner, R.P. Safety and Tolerability of Escalating Doses of House Dust Mite- Peptide Antigen Desensitization (HDM-PAD). J. Allergy Clin. Immunol. 2013, 131, AB37. [Google Scholar] [CrossRef]
- Hafner, R.P.; Couroux, P.; Armstrong, K.; Salapatek, A.M.; Patel, D.; Larché, M. Persistent treatment effect achieved at one year after four doses of Der p derived synthetic peptide immuno-regulatory epitopes in an exposure chamber model of House Dust Mite allergy. J. Allergy Clin. Immunol. 2014, 133, AB289. [Google Scholar] [CrossRef]
- Hafner, R.P.; Salapatek, A.M.; Larché, M.; Ahenkorah, B.; Patel, P.; Pawsey, S. Comparison of the treatment effect of house dust mite synthetic peptides immune-regulatory epitopes in the environmental exposure chamber and field setting two years after a short course of treatment. Allergy 2015, 70, 28. [Google Scholar]
- Spertini, F.; Perrin, Y.; Audran, R.; Pellaton, C.; Boudousquie, C.; Barbier, N.; Thierry, A.C.; Charlon, V.; Reymond, C. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J. Allergy Clin. Immunol. 2014, 134, 239–240. [Google Scholar] [CrossRef]
- Spertini, F.; DellaCorte, G.; Kettner, A.; de Blay, F.; Jacobsen, L.; Jutel, M.; Worm, M.; Charlon, V.; Reymond, C. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: Results of a phase IIb study. J. Allergy Clin. Immunol. 2016, 138, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kettner, A.; DellaCorte, G.; de Blay, F.; Jacobsen, L.; Jutel, M.; Worm, M.; Charlon, V.; Simonsen, K.; Reymond, C.; Spertini, F. Benefit of Bet v 1 contiguous overlapping peptide immunotherapy persists during first follow-up season. J. Allergy Clin. Immunol. 2018, 142, 678–680 e7. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.K.; Frankish, C.W.; Armstrong, K.; Steacy, L.; Tenn, M.W.; Pawsey, S.; Hafner, R.P. Persistence of the clinical effect of grass allergen peptide immunotherapy after the second and third grass pollen seasons. J. Allergy Clin. Immunol. 2020, 145, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Zhernov, Y.; Curin, M.; Khaitov, M.; Karaulov, A.; Valenta, R. Recombinant allergens for immunotherapy: State of the art. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Agache, I. Peptide allergen immunotherapy-unraveling new pathways. J. Allergy Clin. Immunol. 2019, 144, 658–660. [Google Scholar] [CrossRef]
- Rudulier, C.D.; Tonti, E.; James, E.; Kwok, W.W.; Larché, M. Modulation of CRTh2 expression on allergen-specific T cells following peptide immunotherapy. Allergy 2019, 74, 2157–2166. [Google Scholar] [CrossRef]
- Liccardi, G.; D’Amato, M.; D’Amato, G. Oleaceae pollinosis: A review. Int. Arch. Allergy Immunol. 1996, 111, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 27, 1830. [Google Scholar] [CrossRef] [PubMed]
- Hamman-Khalifa, A.M.; Castro, A.J.; Jiménez-López, J.C.; Rodríguez-García, M.I.; Alché, J.D. Olive cultivar origin is a major cause of polymorphism for Ole e 1 pollen allergen. BMC Plant Biol. 2008, 25, 10. [Google Scholar] [CrossRef]
- Castro, A.J.; Bednarczyk, A.; Schaeffer-Reiss, C.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Alché, J.D. Screening of Ole e 1 polymorphism among olive cultivars by peptide mapping and N-glycopeptide analysis. Proteomics 2010, 10, 953–962. [Google Scholar] [CrossRef]
- Lombardero, M.; Obispo, T.; Calabozo, B.; Lezaún, A.; Polo, F.; Barber, D. Cross-reactivity between olive and other species. Role of Ole e 1-related proteins. Allergy 2002, 57 (Suppl. S71), 29–34. [Google Scholar] [CrossRef]
- Palomares, O.; Swoboda, I.; Villalba, M.; Balic, N.; Spitzauer, S.; Rodríguez, R.; Valenta, R. The major allergen of olive pollen Ole e 1 is a diagnostic marker for sensitization to Oleaceae. Int. Arch. Allergy Immunol. 2006, 141, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Rodríguez, R.; Batanero, E. The spectrum of olive pollen allergens. From structures to diagnosis and treatment. Methods 2014, 66, 44–54. [Google Scholar] [CrossRef]
- Oeo-Santos, C.; Mas, S.; Quiralte, J.; Colás, C.; Blanca, M.; Fernández, J.; Feo Brito, F.; Villalba, M.; Barderas, R. A Hypoallergenic Polygalacturonase Isoform from Olive Pollen Is Implicated in Pollen-Pollen Cross-Reactivity. Int. Arch. Allergy Immunol. 2018, 177, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, J.C.; Robles-Bolivar, P.; Lopez-Valverde, F.J.; Lima-Cabello, E.; Kotchoni, S.O.; Alché, J.D. Ole e 13 is the unique food allergen in olive: Structure-functional, substrates docking, and molecular allergenicity comparative analysis. J. Mol. Graph. Model. 2016, 66, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Quiralte, J.; Llanes, E.; Barral, P.; Arias de Saavedra, J.M.; Sáenz de San Pedro, B.; Villalba, M.; Florido, J.F.; Rodríguez, R.; Lahoz, C.; Cárdaba, B. Ole e 2 and Ole e 10: New clinical aspects and genetic restrictions in olive pollen allergy. Allergy 2005, 60, 360–365. [Google Scholar] [CrossRef]
- Barber, D.; de la Torre, F.; Feo, F.; Florido, F.; Guardia, P.; Moreno, C.; Quiralte, J.; Lombardero, M.; Villalba, M.; Salcedo, G.; et al. Understanding patient sensitization profiles in complex pollen areas: A molecular epidemiological study. Allergy 2008, 63, 1550–1558. [Google Scholar] [CrossRef]
- Villalba, M.; Batanero, E.; López-Otín, C.; Sánchez, L.M.; Monsalve, R.I.; González de la Peña, M.A.; Lahoz, C.; Rodríguez, R. The amino acid sequence of Ole e I, the major allergen from olive tree (Olea europaea) pollen. Eur. J. Biochem. 1993, 216, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Batanero, E.; Villalba, M.; Rodríguez, R. Glycosylation site of the major allergen from olive tree pollen. Allergenic implications of the carbohydrate moiety. Mol. Immunol. 1994, 31, 31–37. [Google Scholar] [CrossRef]
- Martín-Orozco, E.; Cárdaba, B.; del Pozo, V.; de Andrés, B.; Villalba, M.; Gallardo, S.; Rodriguez-García, M.I.; Fernández, M.C.; Alché, J.D.; Rodriguez, R. Ole e I: Epitope mapping, cross-reactivity with other Oleaceae pollens and ultrastructural localization. Int. Arch. Allergy Immunol. 1994, 104, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Calzada, D.; Aguerri, M.; Baos, S.; Montaner, D.; Mata, M.; Dopazo, J.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdaba, B. Therapeutic targets for olive pollen allergy defined by gene markers modulated by Ole e 1-derived peptides. Mol. Immunol. 2015, 64, 252–261. [Google Scholar] [CrossRef]
- Calzada, D.; Cremades-Jimeno, L.; Pedro, M.Á.; Baos, S.; Rial, M.; Sastre, J.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdaba, B. Therapeutic potential of peptides from Ole e 1 in olive-pollen allergy. Sci. Rep. 2019, 4, 15942. [Google Scholar] [CrossRef]
