A projected increased use of total joint arthroplasties will naturally result in a related increase in the number of prosthetic joint infections (PJIs). Suppression of the local peri-implant immune response counters efforts to eradicate bacteria, allowing the formation of biofilms and compromising preventive measures taken in the operating room. For these reasons, the prevention of PJI should focus concurrently on the following targets: (i) identifying at-risk patients; (ii) reducing “bacterial load” perioperatively; (iii) creating an antibacterial/ antibiofilm environment at the site of surgery; and (iv) stimulating the local immune response. Despite considerable recent progress made in experimental and clinical research, a large discrepancy persists between proposed and clinically implemented preventative strategies. The ultimate anti-infective strategy lies in an optimal combination of all preventative approaches into a single “clinical pack”, applied rigorously in all settings involving prosthetic joint implantation. In addition, “anti-infective” implants might be a choice in patients who have an increased risk for PJI. However, further progress in the prevention of PJI is not imaginable without a close commitment to using quality improvement tools in combination with continual data mining, reflecting the efficacy of the preventative strategy in a particular clinical setting.
- biomaterial-associated infection
- prosthetic joint infection
- preventative measures
- at-risk patient
- bacterial contamination
- anti-adhesive
- antibacterial surface treatment
- quality improvement
- machine learning
Prosthetic joint infection (PJI) is a disastrous complication of modern orthopedic surgery, frequently leading to prolonged morbidity and even to increased mortality. PJI results from a complex interplay of numerous factors that lead to an inability of periprosthetic immune cells to protect implant surfaces and periprosthetic tissues from bacterial colonization. Why has PJI not been eradicated despite modern preventative measures? First, PJI may be a manifestation of host–bacteria interactions that differ from those traditionally assumed to result from intraoperative colonization of implant surfaces (conceptual limits). Second, there may be a failure to eliminate a significant bacterial load during a surgery (organizational limits). Third, some studies may overestimate the impact of a preventative intervention (scientific limits, biases).
1. Preoperative strategies
Assessment of a patient’s individual risk for PJI is crucial even if the true potential for modifying some risk parameters in individual cases may be limited.
- Identification of at-risk patients
- Preventative strategies for all population/at-risk patients
2. Perioperative strategies
A host skin and people working in the operating room environment are a source of bacteria contaminating surgical wounds, tools, and implants.
- Preparation of operative field
- Operating room—technical parameters, traffic
- Team, personnel
- Systemic/local antibiotics
- Intraoperative care for TJA patients
- Surgeon performance
- Anti-infective implant
3. Postoperative strategies
Postoperative strategies are intended to eliminate risk for PJI associated with bacterial colonization of implant surface/periprosthetic tissues postoperatively.
- Wound care
- Measures against hematogenous and directly spreading PJI
4. Quality improvement
Through many years of practice, a number of strategies have been tested, all aimed at reducing the risk for PJI development, applied pre-, peri-, or postoperatively. Even though their educational impact has been enormous, the overall risk for PJI ranges from 0.5% to higher depending on the study location, design, and/or a particular group of patients who were treated by TJA. We have a relatively solid body of knowledge about “anti-infective” interventions; however, we perhaps fail in implementing these strategies in clinical practice. This situation is nothing new in clinical medicine.
5. Contribution of machine learning to prevention of PJI
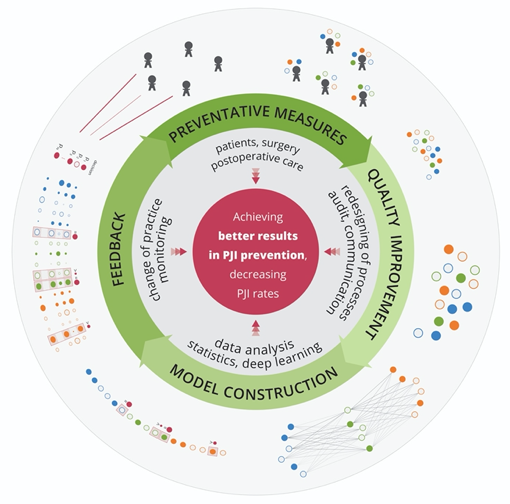
Machine learning references a group of methods for training software algorithms to learn from and act on new data to continuously improve health care performance. Here, we would like to emphasize the potential of deep learning to reduce the number of PJIs by mining new knowledge from the huge amount of data collected for individual patients (Figure) in every country.
6. A step towards precision prevention of PJI?
Precision prevention of PJI describes delivery of the precise preventative content to a particular patient at an optimal time. Theoretically, the preventative interventions should be matched to a particular patient according to the individual preoperatively calculated risk for PJI. For at-risk patients, depending on their estimated risk for PJI, an extra-preventative package could include, e.g., surgery in a special “anti-infective” room, prolonged antibiotic prevention, placement of a local antibacterial carrier around the implant, and implantation of an anti-infective implant.
7. Conclusions
The prevention of PJI is an absolutely essential part of TJA clinical practice because all patients have the right not to be harmed during TJA. When complex preventative measures are fully applied and adhered to in clinical practice, PJI should be preventable in almost all cases. Thus, the secret of clinical success lies in an optimal combination of critical preoperative and perioperative measurements. The optimal preventative configuration has to be continuously designed/redesigned according to the actual rate of PJI and its appropriate analysis. In this sense, prevention will always be a systematic work in progress. The outcomes of such PJI-oriented QI projects have to be monitored and analyzed. If a particular preventative configuration shows excellent efficacy, then it should be implemented, trained on, and controlled widely across hospitals globally. Patients, payors, and regulatory authorities should insist on adherence to these up-to-date preventative standards.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9072190