Heartburn and non-cardiac chest pain are the predominant symptoms in many esophageal disorders, such as gastroesophageal reflux disease (GERD), non-erosive reflux disease (NERD), functional heartburn and chest pain, and eosinophilic esophagitis (EoE). At present, neuronal mechanisms underlying the process of interoceptive signals in the esophagus are still less clear. Noxious stimuli can activate a subpopulation of primary afferent neurons at their nerve terminals in the esophagus. The evoked action potentials are transmitted through both the spinal and vagal pathways to their central terminals, which synapse with the neurons in the central nervous system to induce esophageal nociception. Over the last few decades, progress has been made in our understanding on the peripheral and central neuronal mechanisms of esophageal nociception. In this review, we focus on the roles of capsaicin-sensitive vagal primary afferent nodose and jugular C-fiber neurons in processing nociceptive signals in the esophagus. We briefly compare their distinctive phenotypic features and functional responses to mechanical and chemical stimulations in the esophagus. Then, we summarize activation and/or sensitization effects of acid, inflammatory cells (eosinophils and mast cells), and mediators (ATP, 5-HT, bradykinin, adenosine, S1P) on these two nociceptive C-fiber subtypes. Lastly, we discuss the potential roles of capsaicin-sensitive esophageal afferent nerves in processing esophageal sensation and nociception. A better knowledge of the mechanism of nociceptive signal processes in primary afferent nerves in the esophagus will help to develop novel treatment approaches to relieve esophageal nociceptive symptoms, especially those that are refractory to proton pump inhibitors.
1. Esophageal Interoception and Nociception
Interoception is defined as “the representation of the internal world and includes the processes by which an organism senses, interprets, and regulates signals from within itself”
[1]. The concept of “interoceptive” was first described by Dr. Sherrington over a hundred years ago. He referred to interoceptive as the internal surface of the body in contrast to exteroceptive as the external surface in direct contact with the environment
[2]. A representation of one’s internal world is often initiated by activation of afferent nerves that are sensitive to certain physiological activities in visceral organs that are derived largely from sensory neurons situated mainly in the dorsal root ganglia and vagal sensory ganglia
[1]. Beyond these types of general proclamations, relatively little is known about the afferent nerve subtypes, especially vagal afferents, in mediating interoceptive signals.
In contrast to somatosensation that is exclusively processed by spinal afferent nerves, the internal signals from visceral tissues and organs are processed by both spinal afferents and vagal afferents
[3][4][5]. It is now recognized that vagal afferent nerves are interoceptive nerves that can encode visceral stimulus not only in physiological but also in noxious ranges
[6][7]. A better understanding of interoceptive signaling through primary visceral afferents will improve our knowledge of the pathogenesis of many gastrointestinal sensory/motor dysfunctions.
2. Esophageal Primary Afferent Pathways
Esophageal sensory transduction is initiated by the stimulation of primary afferent neurons at their peripheral nerve terminals, which are distributed in the wall of the esophagus. The evoked action potentials (APs) are transmitted through both spinal and vagal afferent pathways to their central terminals, which synapse with the neurons in the central nervous system
[3][4][5]. Activation of these afferent nerves with non-noxious stimuli can lead to normal sensations of the presence of food or fluid that likely subconsciously trigger peripheral and central reflexes to coordinate motor functions. In contrast, noxious stimulation and stimuli produced at sites of local inflammation can activate nociceptors that could generate pain and nocifensive behavior in order to avoid potential tissue damage.
The afferent fibers in the esophagus comprise low threshold mechanosensitive nerves that exquisitively respond to a mechanical tension. These so-called “tension receptors” are strongly activated by mechanical distention at pressures that are not sensed as pain. They are derived from neurons in the nodose ganglia and are characterized by conduction of action potentials in the Aδ range (about five times faster than that of nociceptive C-fibers), indicative of small, myelinated nerves. The rest of the afferent innervation of the esophagus comprises polymodal unmyelinated C-fibers that have all the characteristics of classically defined nociceptors. As previously mentioned, the esophagus is innervated by at least three distinct subtypes of nociceptive afferent nerves. The characteristics of the nociceptive subtypes depend strictly on the location of their neuronal cell bodies. The nociceptor subtypes include vagal C-fibers derived from cell bodies in the vagal nodose ganglion (vagal placodal C-fibers) and jugular ganglion (vagal neural crest C-fibers)
[7], in addition to C-fibers with cell bodies in the spinal DRG (spinal neural crest C-fibers)
[8]. The jugular and DRG C-fibers innervating the esophagus are both neural crest-derived and share many phenotypic features. Therefore, they are both substantially distinct from the placodal-derived nodose C-fibers. Based on their activation profiles, all three of these nerve subtypes fit Sherrington’s definition of a nociceptor, thereby serving the function to, in Sherrington’s words, “provide the (organ) with a so-to-say sense of its own potential injury”
[2]. The vast majority of nociceptive C-fibers innervating the esophagus express TRPV1 and can be activated by capsaicin
[7][8] (
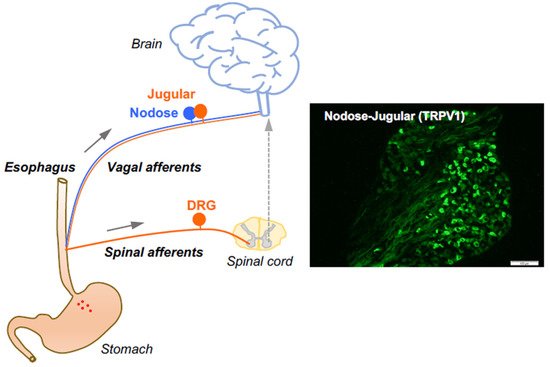
Figure 1). Here, we focus on the roles of capsaicin-sensitive vagal afferent C-fibers in processing esophageal interoceptive signals and their potential contribution to esophageal nociception (heartburn and non-cardiac chest pain).
Figure 1. Esophageal primary afferent pathways. The extrinsic primary afferent nerves in the esophagus include vagal and spinal afferents with their neuronal cell bodies in nodose and jugular ganglia (NJG) and dorsal root ganglia (DRG), respectively. (right: TRPV1 immunoreactivities in mouse NJG, bar size = 100 μM).
3. Capsaicin-Sensitive Vagal Afferent C-Fibers in the Esophagus
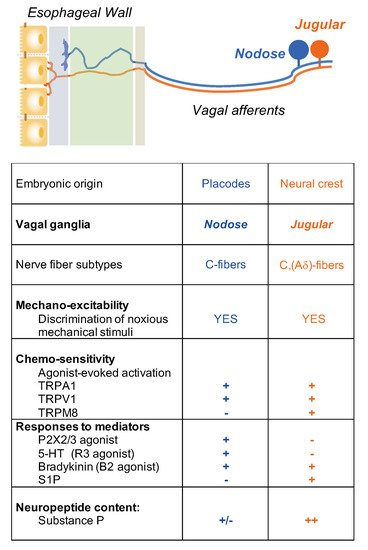
It is critical to define the functional difference between vagal nodose (placode-derived) and jugular (neural crest-derived) neuron populations. This is because these two neuron populations innervate different parts of the CNS. Nodose fibers synapse with neurons in the nucleus tractus of solitarius (NTS) of the brain stem, whereas it has recently been shown that jugular fibers synapse with neurons in the paratrigeminal nucleus, similar to somatosensory nociceptors
[9]. Thus, the subsequently induced sensations and reflexed autonomic efferent activities will depend on whether nodose or jugular C-fibers are activated.
Nociceptive C-fiber neurons can be further divided into subpopulations by their expression of distinctive neuropeptides, receptors, and ion channels that encode specific noxious thermal, mechanical, and chemical stimuli
[10]. For example, tachykinergic C-fibers that innervate the esophagus, which are generally derived from neurons in the DRG and vagal jugular ganglion. Most nodose C-fibers are non-tachykinergic. Therefore, one can argue that any substance P-mediated inflammation, so-called neurogenic inflammation, in the esophagus, is more likely caused by activation of DRG and vagal jugular C-fibers rather than nodose C-fibers.
4. Activation and Sensitization of Esophageal Vagal Nodose and Jugular C-Fibers
Electrophysiological studies have revealed functional distinctions between the two vagal C-fiber subtypes in the esophagus. We have successfully established an ex vivo esophageal-vagal preparation for extracellular single-unit recordings
[7]. In this model, the recording electrode is placed into intact vagal nodose or jugular ganglion, respectively, and the evoked action potential discharges are recorded in a sensory neuron cell soma, while noxious chemical and mechanical stimuli are applied at its nerve terminals in the esophagus. This unique approach enables us to thoroughly characterize esophageal vagal afferent nerve subtypes, to define the key ion channel-mediated activation responses to noxious stimuli, and to reveal the mechanisms of activation and sensitization
[11][12][13][14][15][16] (
Figure 2).
Figure 2. Nociceptive vagal afferent subtypes in the esophagus.
5. Potential Roles of Capsaicin-Sensitive Afferents in Esophageal Disorders
5.1. Eosinophils
Eosinophils, by definition, are elevated in esophageal mucosa in the eosinophilic esophagitis (EoE), as well as other inflammatory esophageal disorders. Eosinophils can release a variety of cationic proteins that may influence afferent C-fiber function in the proximity. It has been shown that eosinophil cationic protein and major basic protein sensitized airway vagal C-fibers. Such effects could be prevented by pretreatment with poly-
l-glutamic acid (PLGA) to neutralize cationic charges
[17][18]. In esophageal nodose C-fibers, perfusion with synthetic cationic protein poly-
l-lysine (PLL) did not evoke action potential discharges but increased their responses to esophageal distension. This potentiation effect could be prevented by pretreatment with PLGA. In contrast to nodose C-fibers, PLL neither induced action potential discharges nor changed the responses to esophageal distension in jugular C-fibers
[19]. The roles of eosinophil granule proteins in the regulation of esophageal vagal afferent nerve mechano-excitabilities deserve further investigation. The sensitization of mechanical activity, in theory, could lead to a situation in which the action potential frequency that is usually interpreted as innocuous by the CNS is now increased to a point where the same innocuous distention is perceived as noxious, setting in motion the process by which the sensations and reflexes become troublesome.
5.2. Mast Cells
Mast cells are strategically distributed in close proximity to afferent nerve terminals in the host-defense surface and play a key role in sensitization of nociceptive C-fibers’ excitability
[6][20]. Moreover, in esophageal allergic disorders, such as EoE, mast cell numbers are increased in the wall of the esophagus. The percentage increase in the number of mast cells can be similar to that of eosinophils. Our recent studies have systematically investigated how IgE-mediated mast cell activation sensitizes esophageal vagal afferent C-fibers, what roles mast cell mediators play, and which ion channels downstream mediate C-fibers’ hyper-excitability. In ovalbumin-sensitized guinea pig esophagus, ovalbumin perfusion selectively activated tissue mast cells to induce histamine release. This significantly enhanced esophageal vagal nodose C-fibers’ excitabilities to both esophageal distension and chemical stimulations. Such sensitization effect was long-lasting even after tissue histamine was washed out and could be prevented by histamine H1 receptor antagonist only before but not after mast cell degranulation
[12]. The possible role of another preformed mast cell mediator, tryptase, has been investigated using the same approach. The study demonstrated that protease-activated receptor-2 (PAR-2)-activating peptide mimicked mast cell activation-induced long-lasting sensitization of esophageal nodose C-fibers. Such effects could be inhibited by pretreatment with TRPA1 inhibitor HC-030031, suggesting that TRPA1 plays a key role downstream to process PAR2-dependent sensitization of nodose C-fibers
[14]. In addition to mast cell preformed mediators, histamine and tryptase, the role of the mast cell lipid mediator prostaglandin D2 (PGD2) in mast cell activation-induced sensitization was further clarified. PGD2 perfusion to nodose C-fiber nerve terminals in the esophagus did not evoke action potential discharges but significantly increased their excitabilities to esophageal distension. Such sensitization effect could be mimicked by prostaglandin D2 DP1 (PGD2 DP1) receptor agonist BW 245C. Pretreatment with PGD2 DP1 receptor antagonist BWA868C inhibited both mast cell activation- and PGD2 perfusion-induced sensitization effects on esophageal nodose C-fibers. Patch clamp recordings indicated that PGD2 could decrease the threshold of action potential discharges in esophageal-labeled nodose neurons. PGD2 perfusion did not activate or sensitize esophageal nodose Aδ-fibers
[15].
5.3. TRPV1 and Acid
TRPV1 in the terminals of esophageal nociceptors can, in theory, participate in the activation of the nerves induced by capsaicin found in foods, endogenous capsaicinoids, acid, heat, and certain G-protein coupled receptors, such as bradykinin. Acid reflux-induced heartburn is the most common symptom in many esophageal disorders. At present, the primary afferent pathways that mediate this noxious stimulation are still less clear. The responses of capsaicin-sensitive vagal afferents to esophageal acid instillation have been investigated in healthy and inflamed esophagi by electrophysiological recordings in animal models. Using extracellular recording ex vivo in guinea pig esophageal-vagal preparations, acid perfusion in healthy esophagi did not evoke action potential discharges in nodose C-fibers but significantly increased their response to esophageal distension, which could be recovered after washing acid out for 90 min. In jugular C-fibers, acid perfusion not only evoked action potential discharges but also inhibited their response to esophageal distension thereafter, which was not recovered after washing out acid for 120 min. Pretreatment with TRPV1 antagonist AMG-9810 inhibited acid-induced effects in nodose and jugular C-fibers
[21]. The different responses to acid between esophageal nodose and jugular C-fibers might result from the differences in their nerve terminal distributions in the esophagus. Esophageal nodose C-fiber nerve endings are mainly distributed in the submucosal layer, while jugular C-fiber nerve terminals are more superficially distributed in the mucosal epithelium
[22]. This morphological feature makes jugular C-fibers more accessible to acid in the esophagus.
5.4. Serotonin (5-Hydroxytrytamine, 5-HT)
5-Hydroxytrytamine (5-HT, serotonin) is one of the most enriched mediators in the gastrointestinal (GI) tract. Its effects on intrinsic enteric neurons and extrinsic vagal and spinal afferents in the GI tract have been extensively investigated and nicely summarized
[23][24]. The effects of 5-HT on esophageal vagal nodose and jugular C-fiber neurons were compared by both extracellular recordings in ex vivo esophageal-vagal preparations and by patch clamp recordings in esophageal-labeled nodose and jugular neurons, respectively. 5-HT selectively activated esophageal nodose but not jugular C-fiber neurons. Such activation effect was mimicked by 5-HT3 receptor agonist 2-methyl-5-HT and could be prevented by 5-HT3 receptor antagonist ondansetron and Y-25130. In addition, 5-HT did not activate esophageal-labeled DRG neurons. The differing responsiveness to 5-HT helps to discriminate placode-derived vagal nodose C-fibers from neural crest-derived vagal jugular and spinal DRG afferent nerves in the esophagus
[11].
5.5. Bradykinin
Bradykinin (BK) is cleaved from the plasma precursor kininogen by kininogenase during inflammation or tissue injury. BK, via its G protein-coupled receptor, not only activates afferent neurons but also sensitizes their response to other stimuli. The effects of BK on esophageal vagal afferent nerve subtypes have been compared and determined. BK activates most esophageal nodose and all jugular C-fibers. This activation is associated with a significant increase in response to esophageal distension. Such effects can be prevented by the BK B2 receptor antagonist WIN64338. TRPA1 agonist AITC activates most BK-positive nodose and jugular C-fibers. Pretreatment with TRPA1 inhibitor HC-030031 prevents BK-induced mechanical hyperexcitabilities but not BK-evoked activation responses in esophageal nodose and jugular C-fibers. In contrast, esophageal vagal nodose Aδ-fibers do not respond to BK or AITC and fail to show mechanical hypersensitivity after BK perfusion
[13].
5.6. Adenosine
Clinical studies demonstrated that intravenous adenosine induced esophageal hyperalgesia by lowering the threshold of pain sensation induced by esophageal distension. Adenosine antagonist attenuated high-dose adenosine-evoked esophageal discomfort and chest pain
[25][26]. These indicate that adenosine may regulate esophageal afferent nerve functions. The possible mechanism has been experimentally addressed. Single-cell RT-PCR analysis of mRNA expression in esophageal-labeled afferent neurons demonstrated that the majority of TRPV1-positive afferent neurons expressed adenosine receptors. The jugular and DRG C-fiber neurons mainly expressed adenosine A1 receptor, and nodose C-fiber neurons expressed adenosine A1 and A2A receptors. A functional study supported these expression profiles and demonstrated that adenosine evoked action potential discharges in both esophageal nodose and jugular C-fibers. Jugular C-fibers could be activated by A1 receptor agonist 2-Chloro-N6-cyclopentyladenosine (CCPA), while nodose C-fiber could be activated by both A1 receptor agonist CCPA and A2A receptor agonist CGS-21680. Similar to most mediators discussed above, adenosine did not activate esophageal nodose Aδ-fibers
[27]. Moreover, adenosine A2A receptor agonist CGS-2168 also significantly increased esophageal distension-evoked action potential discharges in esophageal nodose C-fibers. Such mechanical sensitization effect could be abolished by selective A2A receptor antagonist SCH58261, and by TRPA1 antagonists HC-030031 and AP18
[28].
5.7. S1P
Sphingosine-1-phosphate (S1P) is a metabolite of sphingolipid that is released by different types of cells in the extracellular space at the site of inflammation. S1P has been reported to participate in allergic inflammation-induced airway hypersensitivity through the S1P3 receptor that is expressed in vagal nodose neurons
[29][30]. The effects of S1P on esophageal nodose and jugular C-fibers have been investigated. S1P was able to evoke action potential discharges in jugular C-fibers at their nerve terminals in the esophagus but failed to activate esophageal nodose C-fibers. S1P receptors 1, 2, and 3 were identified in esophageal-labeled nodose and jugular neurons. S1P receptor 1 and 3 agonists each partially mimicked the S1P-elicited effect in jugular C-fibers. S1P did not appear to non-selectively sensitize esophageal nodose or jugular C-fibers to other activating stimuli, such as mechanical distension
[31]. It is of considerable interest to further clarify whether S1P may contribute to esophageal dysfunctions in certain esophageal inflammatory conditions, such as eosinophilic esophagitis.
6. Conclusions
TRPV1 plays an important role in processing noxious stimuli in nociceptive afferent neurons
[32]. TRPV1 can be activated not only by capsaicin but also by acid (and heat), making it a viable target to inhibit acid reflux-induced esophageal nociception. TRPV1 is richly expressed in polymodal afferent C-fibers in the esophagus. There are at least three major subtypes of these afferent nerves, namely vagal nodose C-fibers, vagal jugular C-fibers, and spinal DRG C-fibers. All three subtypes have the characteristics of classical nociceptors. In healthy conditions, they serve to help “sense” the internal environment of the esophagus, where they are somewhat selectively activated by noxious and potentially dangerous stimuli. The consequential action potential discharge signals to the CNS then provide warning sensations, such as pain and heartburn, as well as subconscious autonomic reflexes, such as an increase in secretions. In this manner, these afferent nerves play a role in host defense.
In the inflamed esophagus, tissue-released mediators can induce excessive stimulation of C-fibers. This may lead to peripheral and central sensitization and produce chronic warning sensations of pain/heartburn and reflexes that extend far beyond any useful function. Despite the significant progress that has been made in the management of gastroesophageal reflux symptoms, there are still 30–40% of patients with persistent symptoms that are refractory to proton pump inhibitors
[33][34]. A better understanding of the function of each nociceptor subtype, along with the mechanisms underlying their activation and sensitization, may lead to novel therapeutic strategies aimed at reducing the suffering that accompanies acid reflux disorders. Such understanding may also lead to strategies that more readily allow for the distinction between cardiac and non-cardiac chest pain.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26133929