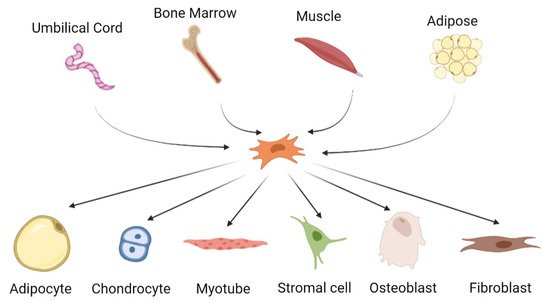
2. Mesenchymal Stem Cells for Cartilage Tissue Engineering
MSCs are multipotent cells that can be differentiated into several types of cells including chondrocytes, adipocytes, osteoblasts and myogenic and neuronal cells [
92,
93,
94,
95,
96] (
Figure 1). MSCs can be isolated from various sources, primarily bone marrow, adipose tissue, dental pulp, placenta and umbilical cord, as well as from the skeletal tissues. They are characterized by their fibroblastic shape and specific marker expressions such as CD11b
+, CD14
−, CD34
−, CD45
−, HLA-DR
−, CD73
+, CD90
+ and CD105
+ [
97].
Figure 1. The origin and differentiation potential of mesenchymal stem cells.
Isolation of MSCs was first reported by Friedenstein and co-workers through their early works in the 1960s and 1970s [
98,
99]. The first isolated MSCs were identified in bone marrow, where the cells showed osteogenic potential and distinguished themselves from the majority of hematopoietic cells by their rapid adherence to tissue culture vessels and fibroblast-like appearance of their progeny in culture. In addition, in vivo transplantation of the cells led to differentiation of the cells into multiple skeletal tissues (bone, cartilage, adipose and fibrous tissues) confirming the multipotential of the cells [
100]. Following the report by Friedenstein and colleagues, Owen and Caplan had demonstrated the presence of non-hematopoietic adult stem cell in the bone marrow in the late 1980s [
101,
102]. Later, the term mesenchymal stem cell was introduced by Caplan in 1991 [
102] through their success in isolating human bone marrow-derived MSCs.
Unlike chondrocyte cells, MSCs are easy to expand in culture and exhibits chondrogenic potential. The use of MSC-differentiated chondrocytes is a promising strategy for cartilage regeneration. Chondrogenic differentiation from MSCs is well documented from various sources and techniques. Differentiated cells display the important cartilage-specific markers such as collagen type II, aggrecan and sulphated proteoglycans [
103]. Similar to other tissue engineering approach, chondrogenic differentiation could be achieved in the presence of inducers, in this case the most established inducer is TGF-β [
103], although other inducers such as bone morphogenetic proteins (BMPs) [
104] and IGF [
105] were also reported. MacKay et al. [
106] used a combination of 100 nM dexamethasone and 10 ng/mL TGF-β3 and successfully induced chondrogenic differentiation of MSCs characterized by ECM with collagen type II, aggrecan and proteoglycan. Enhanced chondrogenic potential of bone marrow MSCs in a presence of combination treatments TGF-β3/BMP-6 and TGF-β3/IGF-1 were also reported [
105]. The importance of TGF-β signaling in chondrogenic development was confirmed in a study which showed adipose tissue-derived MSCs that did not express TGF-receptor-1 protein had a lower chondrogenic capacity [
107]. Progress in the chondrogenic differentiation potential of MSCs has led to the advancement of cultivation of the cells. MSCs co-cultured with juvenile articular chondrocytes (ACs) [
108] with a presence of TGF-β3 in lower concentration [
109,
110] resulted in efficient chondrogenic differentiation.
Given their highly chondrogenic potential in in vitro culture, MSC-based therapy is among the promising therapeutic approaches to treat OA. Pre-clinical studies demonstrated encouraging data on therapeutic potential of MSCs in OA animal models. Bone marrow MSCs were implanted onto osteochondral defect which artificially made on 16 rabbits. MSCs-implanted rabbits demonstrated improved histological scores as well as enhanced production of collagen type II in the matrix [
111]. Other pre-clinical studies showed the success of intra-articular injections of MSCs in goat [
112] and porcine [
113] models with improved cartilage healing of chondral defects.
In 2002, Wakitani and colleagues transplanted bone marrow MSCs into the articular cartilage defects in knees of 12 patients [
114]. Even though there was no significant clinical improvement after six months, arthroscopic and histological grading scores were better than the control group. This study somehow highlighted the availability of autologous MSC culture thus sparked for more research using the cells. Currently, 74 clinical studies in various phases involving MSCs for OA are referenced at ClinicalTrials.gov. Centeno and colleagues [
115] described percutaneous injection of bone marrow MSC which resulted in significant cartilage growth, decreased pain and increased joint mobility in the patient. Later, they published a case study of 339 patients, showing that of those patients who needed total knee replacement surgery (69% of the patient group), only 6.9% required replacement surgery again following MSC treatment. The study reported that 60% of patients showed >50% pain relief, while 40% reported >75% pain relief at 11 months [
116]. In a randomized controlled trial, 30 patients with persistent knee pain who had not responded to conservative therapies improved in several functional indices and cartilage quality after intra-articular injections of bone marrow MSCs [
117]. In a Phase II clinical trial of allogeneic MSCs, the safety and efficacy of intra-articular injection of Stempeucel
® in 60 patients with OA of knee were determined [
118]. This study found that allogeneic transplant of Stempeucel
® was safe, with improved outcome in pain management scores in the lowest dosage (25 million cells). Regardless of its safety and efficacy, intra-articular injection of Stempeucel
® especially in the highest dosage (150 million cells) exhibited some adverse effects in the patients, but the adverse effects completely recovered upon symptomatic treatment.
Another source of MSCs that is also utilized for OA treatment is adipose-derived MSCs (ADSCs). As reviewed by Hurley [
119], there were 16 studies that reported the use of ADSCs for the treatment of OA with various approaches. ADSCs harvested from infrapatellar fat pad prepared in platelet-rich plasma (PRP) injected into the OA knee showed improved mobility and function and reduced pain scores with no adverse effects [
120]. At two years follow-up, patients had significantly improved pain scores as well as cartilage regeneration as confirmed by MRI [
121]. In a study by Bui et al., non-expanded stromal vascular fraction (SVF) isolated from the adipose tissue and prepared in PRP had been delivered into 21 patients with grade II and III OA and reported significant improvements in pain score as well as increased thickness of the cartilage layer [
122]. Similar procedure and outcome had been described by Bansal et al., which reported reduction in pain levels after 3 months injection with SVF prepared in PRP [
123]. More importantly, autologous ADSCs transplantation reported in these studies offered minimal risk of side effects without graft rejection or tumorigenesis in the recipients, thus provide promising approach for OA treatment.
MSC-derived extracellular vesicles (EVs) are a diverse population of heterogeneous membranous vesicles and enriched in many bioactive molecules such as lipids, proteins, mRNAs, transfer RNA (tRNA), long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and mitochondrial DNA (mtDNA) [
124]. These molecules establish an EVs-mediated transport system which important in intercellular communication to regulate a wide range of physiological and pathological processes and pathways [
125]. MSC-derived EVs have been widely documented to play important roles in the regulation of numerous cell activities such as cell proliferation, differentiation, migration and extracellular matrix synthesis [
126,
127,
128]. When MSCs produce EVs, they encapsulate nucleic acids, proteins, and lipids from donor cells and transfer them to recipient cells such as resting stem cells in the stem cell niche or injured cells in the traumatic microenvironment [
129,
130]. These EVs-cell communications will stimulate regeneration by activating resting stem cells or restoring the functionality of the injured cells. EVs that have been shed by MSCs exhibit similar properties such as functional tissue repair and regeneration as their cells of origin and some studies reported added beneficial effects of MSC-derived EVs [
131,
132]. Moreover, several reports have demonstrated that MSC-derived EVs showed promising findings on cartilage repair and regeneration by regulating immunomodulatory activity, promoting regenerative capacities, diminishing apoptosis, and increasing proliferation [
54,
74,
133,
134].
Exosome derived from human embryonic stem cell-induced MSCs (ESC-MSCs) exhibited remarkable cartilage regeneration in osteochondral defects rat which characterized by complete restoration of hyaline cartilage [
74]. Another study reported by Wang et al. also demonstrated that exosome derived from ESC-MSCs alleviated cartilage destruction and matrix degradation in the destabilization of the medial meniscus (DMM) model by increasing collagen type II (ColII) and aggrecan expressions but reducing ADMTS5 expression as well as improved the maximal and total OARSI scores which resulted in milder OA pathology [
135]. Similar findings were demonstrated by Cosenza et al. in their in vitro and in vivo models [
136]. Exosomes and microparticles isolated from BM-MSC increased anabolic cartilage markers (collagen type II, aggrecan) expression in OA-like chondrocytes in a dose-dependent manner, inhibited catabolic (MMP-13, ADAMTS5) and inflammatory (iNOS) markers, and decreased articular cartilage damage and subchondral bone degradation. Furthermore, EVs isolated from human adipose-derived MSC increased the proliferation and migration of human OA chondrocytes in vitro and decreased the progression of OA and protected cartilage from degeneration in both the monosodium iodoacetate (MIA) rat and the surgical destabilization of the medial meniscus (DMM) mouse models [
133]. Another recent study found that exosomes derived from 3D culture of umbilical MSCs had better chondroprotective effects than exosomes derived from 2D culture systems, significantly stimulating chondrocyte proliferation, migration, and matrix synthesis, as well as improving gross appearance and attenuating cartilage defect in the animal model [
137]. To date, no clinical trial has been conducted using MSC-derived EVs on osteoarthritis.
3. MSCs for the Management of Inflammation in OA
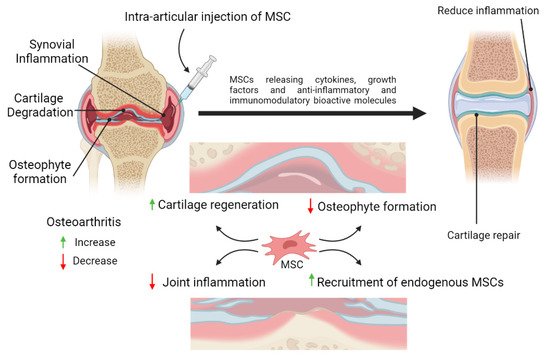
Besides having excellent properties for regeneration of tissues, the immunomodulatory properties of MSCs is also one of their superior characteristics. This makes MSCs as a promising cell source to repair the damage of cartilage tissue and at the same time provide immunomodulatory effect to reduce inflammation in OA. MSCs have been extensively studied for their roles in inflammation. MSCs response to inflammation by homing to the damaged tissues, regulating immune and inflammatory responses at the inflamed areas, thus facilitating repair of the damaged tissues (Figure 2).
Figure 2. Schematic representation of the potential therapeutic strategies utilizing mesenchymal stem cells (MSCs) therapy for cartilage repair and regeneration. MSCs possess anti-inflammatory and immunomodulatory properties which could reduce inflammation in the joint. MSCs may also assist in the healing process by differentiating into chondrocytes or promoting the proliferation and differentiation of the remaining healthy chondroprogenitors into mature chondrocytes, or both. By releasing trophic factors and cell-to-cell interactions, MSCs may enhance cartilage regeneration and reduce synovial inflammation in the osteoarthritic joint.
In general, MSCs have the capacity to modulate both innate and adaptive immune responses. MSCs have been reported to modulate cytokine production by the dendritic cell and Th1/Th2 cells [
138], block maturation and activation of antigen presenting cell (APC) [
139], as well as regulate the production of CD4
+CD25
+regulatory cells [
140]. MSCs are also prominent for their immunosuppressive effects through the inhibition of T-lymphocyte activation and proliferation as well as modulating the expression of pro-inflammatory cytokines and chemokines [
141,
142]. In addition, immunomodulation by MSCs is reported to be mediated via both direct cell to cell contact and also through secretion of soluble factors such as PGE2, indoleamine 2,3-dioxygenase (IDO) and NO [
143]. These aforementioned mechanisms could contribute to resolution of inflammation in OA. However, it is still unclear how MSCs facilitate tissue regeneration and inflammation process. Many studies have shown that MSCs’ paracrine activity may play some role in modifying the milieu of the injured tissue, resulting in more favorable circumstances for tissue regeneration [
67]. MSCs secrete cytokines to reduce inflammation in surrounding tissues and initiate cartilage repair, which is followed by chondrogenic proliferation and the secretion of ECM proteases and growth factors such as TGF-β IGF-1 and FGF [
144].
Intra-articular administration of MSCs into arthritic shoulder of the rat model indicated downregulation of ADAMTS5 expression in the joint cartilage, but increased expression of TNF-α stimulated gene/protein 6 (TSG-6) and inhibited the expression of anti-calcitonin gene related peptide (CGRP) indicating suppression of the central sensitization of pain [
145]. Another study using intra-articular administration of umbilical cord-derived MSCs (UC-MSCs) indicated anti-inflammatory and anti-catabolic effects of UC-MSCs as demonstrated by decreased expression of the pro-inflammatory cytokines and MMPs in the synoviocytes of the rabbit model [
146].
Many in vitro and in vivo investigations have shown that MSC-derived EVs have significant anti-inflammatory and regenerative effects in OA models. In an experimental study by Vonk et al., EV isolated from human bone marrow MSC altered the TNF-α-mediated upregulation of COX2 and pro-inflammatory interleukins, i.e., IL-1α, IL-1β, IL-6, IL-8 and IL-17 when co-cultured with TNF-α-stimulated OA chondrocyte [
147]. EVs derived from human AD-MSCs demonstrated chondroprotective effects by decreasing the release of inflammatory mediators (e.g., TNF-, IL-6, PGE2 and NO) and MMP activity, while increasing the production of the anti-inflammatory cytokine IL-10. In a recent study, exosomes from embryonic MSCs reduced inflammatory response, enhanced cartilage repair and subchondral bone healing, reversed IL-1-mediated inhibition of sulfated glycosaminoglycan synthesis, and decreased IL-1-induced production of nitric oxide and MMP-13 via adenosine-mediated activation of AKT, ERK and AMPK pathways in an OA model of the temporomandibular joint of immunocompetent rats [
54]. The immunomodulatory properties of MSCs and MSC-derived EVs may help decrease inflammation and prevent the progression of OA, making them potential therapeutic sources for OA.