Biomass provides a wealth of renewable and bio-waste resources for bioplastics synthesis. Many of these bio-based plastics, encompass capacities for biodegradation and bioprocessing with high performance features akin to petroleum-based plastics. The realisation of bioplastics that exhibit a complete set of mechanical and biodegradability, hold the promise of delivering material of ecologically sustainable, low carbon footprint circularity.
- biomaterials
- biodegradation
- bioplastics
- mechanical performance
- barrier performance
- processability
1. Introduction
2. Bioplastic Production
3. Biosynthesised Plastics
| 3-Hidroxyacids | Structure |
|---|---|
| butyric (3HB) | 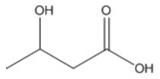 |
| hexanoic (3HHx) | 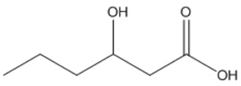 |
| octanoic (3HO) | 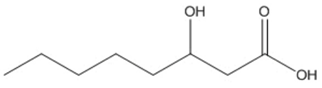 |
| decanoic (3HD) | 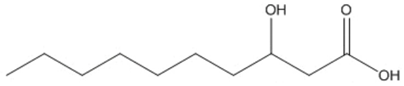 |
| dodecanoic (3HDD) | 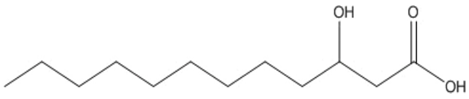 |
| Polymer | Structure |
|---|---|
| poly(6-hydroxyhexanoate) | 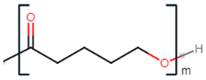 |
| poly(3-hydroxyoctanoate) | 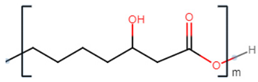 |
| monomethoxy-terminated poly(ethylene glycol) (mPEG) | 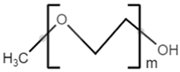 |
| poly(ethylene glycol) | 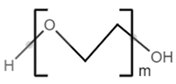 |
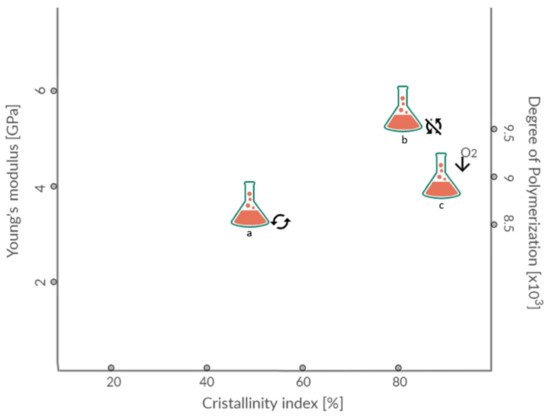
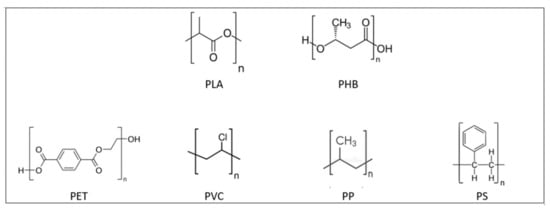
4. Bioplastic Mechanical Performance
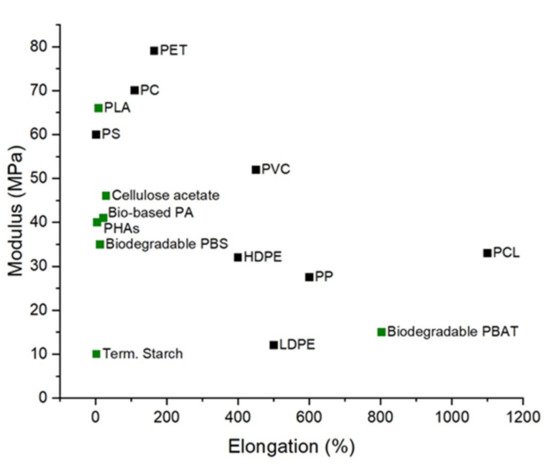
5. Bioplastic Barrier Performance
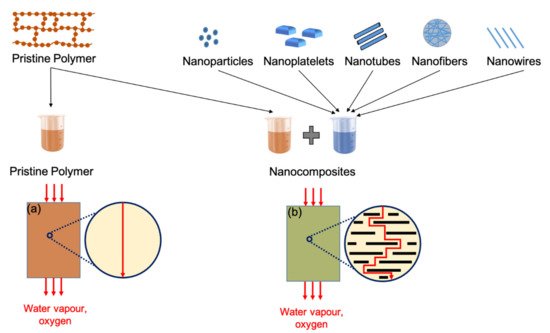
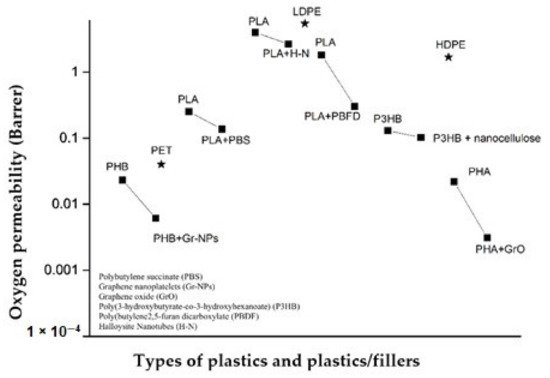
6. Bioplastic Processing and Formulation
6.1. Blends and Composites
6.2. Compatibilisers and Plasticisers
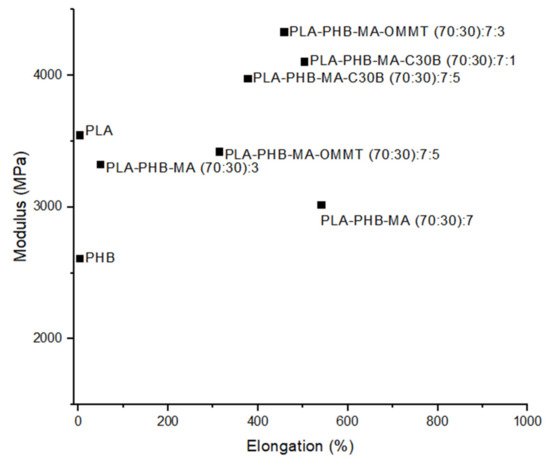
6.3. Natural Fillers
| Process | Natural Fibre Used | Biopolymer Matrix | Outcomes | Ref. | ||
|---|---|---|---|---|---|---|
| Interfacial Adhesion | Mechanical Properties | Barrier Properties | ||||
| Treatment with compatibilisers; Polyglycerol polyglycidyl ether (SR-4GL), Trimethylol propane polyglycidyl ether (SR-TMP), and (Polyglycerol polypropyleneoxide (SC-P1000) | Cellulose fibres | PLA | Improved interfacial adhesion between fibres and PLA and | Inhibited degradation of the PLA matrix | [65] | |
| STEFAC TM 8170, surfactant modification | Cellulose fibres | PLA/PHB | Enhanced mechanical performance | Improved water resistance, reduced oxygen and UV-light transmission, as well as appropriate disintegration in compost | [94] | |
| Alkali treatment | Kenaf fibre | PHB | Reduction in the crystallinity of PHB (up to 6% reduction), making it more ductile, and improvement of the flexural modulus by up to 11%. | [75] | ||
| Silane treatment | Flax fibre | PLA | Improvement to fibre/matrix adhesion with 2% w/w silane content, yet further improvement of the fibre-matrix interface can be partially resolved by silane/alkali treatment combination. | Improved mechanical properties | [80] | |
| Alkali treatment | Flax fibres | PLA | Tg values of fabricated bio-composites were lowered by 10 °C for 10% NaOH treatment and 15 °C for 30% NaOH treatment | [82] | ||
| Treatment with ethylene plasma | Flax fibres | PHB | Improved interfacial adhesion strength in the bio-composite | Improved thermal resistance | [88] | |
6.4. Bio-Coatings
The application of bio-based coatings to biocomposites and natural fibre reinforced biocomposites is a promising approach proposed to overcome the significant water uptake propensities of natural fibres and increase the moisture resistance of bioplastics for fluid barrier property application requirements. Exposure to long term environmental/hygroscopic ageing necessitates the induction of a higher level of hydrophobicity in chemically modified natural fibre reinforced bio-composites and bioplastics in general. Introducing bio-based coatings to natural fibres reinforced biocomposites, ensures the environmentally friendly and biodegradable nature of fibres. Besides, bio-based coatings are obtained from renewable resources and have superior hydrophobic characteristics [62]. For instance, polyurethane (PU) coatings were first introduced as bio-based coating resins in the 1950s [98]. PU coatings mainly provide their composites with high solvent resistance, hydrolytic stability, resistance to acid–base conditions and weather-ability [62]. Currently, most of the industrially produced PUs are petroleum-based polyols. Thus, renewable resources, such as vegetable oil [99][100][101], canola oil [102], soybean oil [103][104][105] and castor oil [106][107][108], are thoroughly investigated and were able to produce PU coating of competing properties to that of petroleum-based ones.
This entry is adapted from the peer-reviewed paper 10.3390/polym13132155
References
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53.
- Koller, M.; Maršálek, L.; Dias, M.M.D.S.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38.
- Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxy-alkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30.
- Rajwade, J.M.; Paknikar, K.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511.
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555.
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323.
- Ciesielski, S.; Mozejko-Ciesielska, J.; Pisutpaisal, N. Plant oils as promising substrates for polyhydroxyalkanoates pro-duction. J. Clean. Prod. 2015, 106, 408–421.
- Shuai, X.-T.; Jedlinski, Z.; Luo, Q.; Farhod, N. Synthesis of novel block copolymers of poly(3-hydroxybutyric acid) with poly(ethylene glycol) through anionic polymerisation. Chin. J. Polym. Sci. 2000, 18, 19–23.
- Fu, J.; Sharma, P.; Spicer, V.; Krokhin, O.V.; Zhang, X.; Fristensky, B.; Wilkins, J.A.; Cicek, N.; Sparling, R.; Levin, D.B. Effects of impurities in biodiesel-derived glycerol on growth and expression of heavy metal ion homeostasis genes and gene products in Pseudomonas putida LS46. Appl. Microbiol. Biotechnol. 2015, 99, 5583–5592.
- Noda, I.; Lindsey, S.B.; Caraway, D. Nodax™ Class PHA Copolymers: Their Properties and Applications. In Beneficial Microorganisms in Food and Nutraceuticals; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 237–255.
- Kunioka, M.; Doi, Y. Thermal degradation of microbial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 1933–1936.
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545.
- Qiu, Y.-Z.; Han, J.; Guo, J.-J.; Chen, G.-Q. Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Gluconate and Glucose by Recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol. Lett. 2005, 27, 1381–1386.
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908.
- Ravenelle, F.; Marchessault, R.H. One-Step Synthesis of Amphiphilic Diblock Copolymers from Bacterial Poly([R]-3-hydroxybutyric acid). Biomacromolecules 2002, 3, 1057–1064.
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Pro-duction. Front. Microbiol. 2019, 10, 992.
- Colombo, B.; Favini, F.; Scaglia, B.; Sciarria, T.P.; D’Imporzano, G.; Pognani, M.; Alekseeva, A.; Eisele, G.; Cosentino, C.; Adani, F. Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnol. Biofuels 2017, 10, 1–15.
- Rajendran, N.; Sharanya, P.; Sneha Raj, M.; Ruth Angeeleena, B.; Rajam, C. Seaweeds can be a new source for bio-plastics. J. Pharm. Res. 2012, 5, 1476–1479.
- Chee, J.Y.; Tan, Y.; Samian, R.; Sudesh, K. Isolation and Characterization of a Burkholderia sp. USM (JCM15050) Capable of Producing Polyhydroxyalkanoate (PHA) from Triglycerides, Fatty Acids and Glycerols. J. Polym. Environ. 2010, 18, 584–592.
- Koller, M.; Salerno, A.; Dias, M.; Reiterer, A.; Braunegg, G. Modern biotechnological polymer synthesis: A review. Food Technol. Biotechnol. 2010, 48, 255–269.
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84.
- Sindhu, R.; Pandey, A.; Binod, P. Solid-state fermentation for the production of Poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181.
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals. Crit. Rev. Biotechnol. 2017, 37, 510–524.
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.; Spencer-Milnes, X.; Garcia, L.D.A.; Paschou, D.; Lazenbatt, C.; Kong, D.; et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440.
- Liu, M.; Li, S.; Xie, Y.; Jia, S.; Hou, Y.; Zou, Y.; Zhong, C. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2018, 102, 1155–1165.
- Hungund, B.; Gupta, S. Strain improvement of Gluconacetobacter xylinus NCIM 2526 for bacterial cellulose production. Afr. J. Biotechnol. 2010, 9, 32.
- Neves, N.M.; Reis, R.L. (Eds.) Biomaterials from Nature for Advanced Devices and Therapies; Wiley: Hoboken, NJ, USA, 2016; pp. 384–399.
- Volova, T.G.; Prudnikova, S.; Sukovatyi, A.G.; Shishatskaya, E. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428.
- Lin, S.-P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219.
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598.
- Hsieh, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J. Taiwan Inst. Chem. Eng. 2016, 63, 46–51.
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulose composites: Synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 2015, 10, 1847–1861.
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komaga-taeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581.
- Parte, F.G.B.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414.
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864.
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An Update. Environ. Sci. Technol. 2020, 54, 4712–4732.
- Chen, S.-Q.; Lopez-Sanchez, P.; Wang, D.; Mikkelsen, D.; Gidley, M.J. Mechanical properties of bacterial cellulose synthesised by diverse strains of the genus Komagataeibacter. Food Hydrocoll. 2018, 81, 87–95.
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70.
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread appli-cations—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392.
- Visakh, P.M. CHAPTER 1. Polyhydroxyalkanoates (PHAs), their Blends, Composites and Nanocomposites: State of the Art, New Challenges and Opportunities. In Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites; Green Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–17.
- Goh, L.-K.; Purama, R.K.; Sudesh, K. Enhancement of Stress Tolerance in the Polyhydroxyalkanoate Producers without Mobilization of the Accumulated Granules. Appl. Biochem. Biotechnol. 2014, 172, 1585–1598.
- Dos Santos, A.J.; Valentina, L.V.O.D.; Schulz, A.A.H.; Duarte, M.A.T. From Obtaining to Degradation of PHB:Material Properties. Part I. Ing. Cienc. 2017, 13, 269–298.
- El-Hadi, A.M.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674.
- Smith, M.K.M.; Paleri, D.M.; Abdelwahab, M.; Mielewski, D.F.; Misra, M.; Mohanty, A.K. Sustainable composites from poly(3-hydroxybutyrate) (PHB) bioplastic and agave natural fibre. Green Chem. 2020, 22, 3906–3916.
- Barham, P.J.; Keller, A.; Otun, E.L.; Wills, H.H.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hyd roxybutyrate. J. Mater. Sci. 1984, 19.
- Barham, P.J.; Keller, A. The relationship between microstructure and mode of fracture in polyhydroxybutyrate. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 69–77.
- Janigová, I.; Lacıík, I.; Chodák, I. Thermal degradation of plasticized poly(3-hydroxybutyrate) investigated by DSC. Polym. Degrad. Stab. 2002, 77, 35–41.
- Shim, S.H.; Kim, K.T.; Lee, J.U.; Jo, W.H. Facile Method to Functionalize Graphene Oxide and Its Application to Poly(ethylene terephthalate)/Graphene Composite. ACS Appl. Mater. Interfaces 2012, 4, 4184–4191.
- Papadopoulou, E.L.; Basnett, P.; Paul, U.C.; Marras, S.; Ceseracciu, L.; Roy, I.; Athanassiou, A. Green Composites of Poly(3-hydroxybutyrate) Containing Graphene Nanoplatelets with Desirable Electrical Conductivity and Oxygen Barrier Properties. ACS Omega 2019, 4, 19746–19755.
- Dhar, P.; Gaur, S.S.; Soundararajan, N.; Gupta, A.; Bhasney, S.M.; Milli, M.; Kumar, A.; Katiyar, V. Reactive Extrusion of Polylactic Acid/Cellulose Nanocrystal Films for Food Packaging Applications: Influence of Filler Type on Thermomechanical, Rheological, and Barrier Properties. Ind. Eng. Chem. Res. 2017, 56, 4718–4735.
- Azeem, M.; Jan, R.; Farrukh, S.; Hussain, A. Improving gas barrier properties with boron nitride nanosheets in poly-mer-composites. Results Phys. 2019, 12, 1535–1541.
- Bharadwaj, R.K. Modeling the Barrier Properties of Polymer-Layered Silicate Nanocomposites. Macromolecules 2001, 34, 9189–9192.
- Moggridge, G.; Lape, N.K.; Yang, C.; Cussler, E. Barrier films using flakes and reactive additives. Prog. Org. Coat. 2003, 46, 231–240.
- Cui, Z.; Martinez, A.P.; Adamson, D.H. PMMA functionalized boron nitride sheets as nanofillers. Nanoscale 2015, 7, 10193–10197.
- Gitari, B.; Chang, B.P.; Misra, M.; Navabi, A.; Mohanty, A.K. A comparative study on the mechanical, thermal, and water barrier properties of PLA nanocomposite films prepared with bacterial nanocellulose and cellulose nanofibrils. BioResources 2019, 14, 1867–1889.
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Lemstra, P.J.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864.
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Morphology and Thermal Properties of Renewable Resource-Based Polymer Blend Nanocomposites Influenced by a Reactive Compatibilizer. ACS Sustain. Chem. Eng. 2013, 2, 377–386.
- Kick, T.; Grethe, T.; Mahltig, B. A Natural Based Method for Hydrophobic Treatment of Natural Fiber Material. Acta Chim. Slov. 2017, 64, 373–380.
- Lu, N.; Oza, S.; Tajabadi, M.G. Surface Modification of Natural Fibers for Reinforcement in Polymeric Composites. In Surface Modification of Biopolymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 224–237.
- Zhijiang, C.; Yi, X.; Haizheng, Y.; Jia, J.; Liu, Y. Poly(hydroxybutyrate)/cellulose acetate blend nanofiber scaffolds: Prepa-ration, characterization and cytocompatibility. Mater. Sci. Eng. C 2016, 58, 757–767.
- Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R. Thermal and mechanical properties of poly(3-hydroxybutyrate) reinforced with cellulose fibers from wood waste. Ind. Crop. Prod. 2020, 145, 112071.
- Mokhothu, T.H.; John, M.J. Review on hygroscopic aging of cellulose fibres and their biocomposites. Carbohydr. Polym. 2015, 131, 337–354.
- Joffe, R.; Pupure, L.; Berthold, F.; Varna, J. Micro-structure and Mechanical Properties in PLA Reinforced with Cellulosic Fiber Sheets Made by Wet Forming Method. In Proceedings of the 8th International Conference on Composites Testing and Model Identification (CompTest 2017), Leuven, Belgium, 5–7 April 2017.
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable packaging materials conception based on starch and polylactic acid (PLA) reinforced with cellulose. Environ. Sci. Pollut. Res. 2016, 23, 20904–20914.
- Kyutoku, H.; Maeda, N.; Sakamoto, H.; Nishimura, H.; Yamada, K. Effect of surface treatment of cellulose fiber (CF) on durability of PLA/CF bio-composites. Carbohydr. Polym. 2019, 203, 95–102.
- Piekarska, K.; Sowinski, P.; Piorkowska, E.; Haque, M.M.U.; Pracella, M. Structure and properties of hybrid PLA nano-composites with inorganic nanofillers and cellulose fibers. Compos. Part A Appl. Sci. Manuf. 2016, 82, 34–41.
- Hu, R.; Lim, J.-K. Fabrication and Mechanical Properties of Completely Biodegradable Hemp Fiber Reinforced Polylactic Acid Composites. J. Compos. Mater. 2007, 41, 1655–1669.
- Pommet, M.; Juntaro, J.; Heng, J.; Mantalaris, A.; Lee, A.; Wilson, K.; Kalinka, G.; Shaffer, M.S.P.; Bismarck, A. Surface Modification of Natural Fibers Using Bacteria: Depositing Bacterial Cellulose onto Natural Fibers To Create Hierarchical Fiber Reinforced Nanocomposites. Biomacromolecules 2008, 9, 1643–1651.
- Xu, Z.; Yang, L.; Ni, Q.; Ruan, F.; Wang, H. Fabrication of high-performance green hemp/polylactic acid fibre composites. J. Eng. Fibers Fabr. 2019, 14.
- Michel, A.; Billington, S. Characterization of poly-hydroxybutyrate films and hemp fiber reinforced composites exposed to accelerated weathering. Polym. Degrad. Stab. 2012, 97, 870–878.
- Keller, A. Compounding and mechanical properties of biodegradable hemp fibre composites. Compos. Sci. Technol. 2003, 63, 1307–1316.
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Characterisation of hemp fibre reinforced Poly(Lactic Acid) composites. Int. J. Mater. Prod. Technol. 2009, 36, 229.
- Smoca, A.; Kukle, S.; Zelca, Z. Properties of Hemp Fibres Reinforced PLA Composites. Key Eng. Mater. 2019, 800, 205–209.
- Avella, M.; Buzõarovska, A.; Errico, M.E.; Gentile, G.; Grozdanov, A.; Bogoeva-Gaceva, G. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-based biocomposites reinforced with kenaf fibers. J. Appl. Polym. Sci. 2007, 104, 3192–3200.
- Hassan, A.; Isa, M.R.M.; Ishak, Z.A.M. Improving thermal and mechanical properties of injection moulded Kenaf Fibre-reinforced Polyhydroxy-butyrate composites through fibre surface treatment. BioResources 2019, 14, 3101–3116.
- Buzarovska, A.; Bogoeva-Gaceva, G.; Grozdanov, A.; Avella, M. Crystallization behavior of polyhydroxybutyrate in model composites with kenaf fibers. J. Appl. Polym. Sci. 2006, 102, 804–809.
- Graupner, N.; Müssig, J. A comparison of the mechanical characteristics of kenaf and lyocell fibre reinforced poly(lactic acid) (PLA) and poly(3-hydroxybutyrate) (PHB) composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2010–2019.
- Kuciel, S.; Liber-Knec, A. Biocomposites based on PHB filled with wood or kenaf fibers. Polimery 2011, 56, 218–223.
- Bayart, M.; Gauvin, F.; Foruzanmehr, M.R.; Elkoun, S.; Robert, M. Mechanical and moisture absorption characterization of PLA composites reinforced with nano-coated flax fibers. Fibers Polym. 2017, 18, 1288–1295.
- Georgiopoulos, P.; Kontou, E.; Georgousis, G. Effect of silane treatment loading on the flexural properties of PLA/flax unidirectional composites. Compos. Commun. 2018, 10, 6–10.
- Karsli, N.G.; Aytac, A. Properties of alkali treated short flax fiber reinforced poly(lactic acid)/polycarbonate composites. Fibers Polym. 2014, 15, 2607–2612.
- Aydın, M.; Tozlu, H.; Kemaloglu, S.; Aytac, A.; Ozkoc, G. Effects of Alkali Treatment on the Properties of Short Flax Fi-ber-Poly(Lactic Acid) Eco-Composites. J. Polym. Environ. 2011, 19, 11–17.
- Shanks, R.A.; Hodzic, A.; Ridderhof, D. Composites of poly(lactic acid) with flax fibers modified by interstitial polymeri-zation. J. Appl. Polym. Sci. 2006, 99, 2305–2313.
- Foruzanmehr, M.; Vuillaume, P.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304.
- Ventura, H.; Claramunt, J.; Rodríguez-Pérez, M.; Ardanuy, M. Effects of hydrothermal aging on the water uptake and tensile properties of PHB/flax fabric biocomposites. Polym. Degrad. Stab. 2017, 142, 129–138.
- Chilali, A.; Assarar, M.; Zouari, W.; Kebir, H.; Ayad, R. Mechanical characterization and damage events of flax fabric-reinforced biopolymer composites. Polym. Polym. Compos. 2019, 28, 631–644.
- Rytlewski, P.; Stepczyńska, M.; Moraczewski, K.; Malinowski, R.; Karasiewicz, T.; Sikorska, W.; Żenkiewicz, M. Flax fibers reinforced polycaprolactone modified by triallyl isocyanurate and electron radiation. Polym. Compos. 2019, 40, 481–488.
- Lee, S.G.; Choi, S.-S.; Park, W.H.; Cho, D. Characterization of surface modified flax fibers and their biocomposites with PHB. Macromol. Symp. 2003, 197, 089–100.
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008.
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79.
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Express Polym. Lett. 2015, 9, 583–596.
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT 2015, 64, 980–988.
- Abdelwahab, M.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828.
- Arrieta, M.P.; Samper, M.D.; Lopez, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470.
- Rodrigues, J.A.F.R.; Parra, D.F.; Lugão, A.B. Crystallization on films of PHB/PEG blends. J. Therm. Anal. Calorim. 2005, 79, 379–381.
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2018, 47, 2153–2183.
- Lalit, R.; Mayank, P.; Ankur, K. Natural Fibers and Biopolymers Characterization: A Future Potential Composite Material. Stroj. Cas. 2018, 68, 33–50.
- Awasthi, S.; Agarwal, D. Influence of cycloaliphatic compounds on the properties of polyurethane coatings. J. Coat. Technol. Res. 2007, 4, 67–73.
- Alagi, P.; Choi, Y.J.; Hong, S.C. Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur. Polym. J. 2016, 78, 46–60.
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704.
- Deka, H.; Karak, N. Bio-based hyperbranched polyurethanes for surface coating applications. Prog. Org. Coat. 2009, 66, 192–198.
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106.
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846.
- Ismail, E.A.; Motawie, A.; Sadek, E.; Ismail, E.A.; Motawie, A.; Sadek, E. Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt. J. Pet. 2011, 20, 1–8.
- Datta, J.; Głowińska, E. Effect of hydroxylated soybean oil and bio-based propanediol on the structure and thermal properties of synthesized bio-polyurethanes. Ind. Crop. Prod. 2014, 61, 84–91.
- Fu, C.; Zheng, Z.; Yang, Z.; Chen, Y.; Shen, L. A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material. Prog. Org. Coat. 2014, 77, 53–60.
- Thakur, S.; Karak, N. Castor oil-based hyperbranched polyurethanes as advanced surface coating materials. Prog. Org. Coat. 2013, 76, 157–164.
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48.
- Mokhothu, T.H.; John, M.J. Bio-based coatings for reducing water sorption in natural fibre reinforced composites. Sci. Rep. 2017, 7, 1–8.