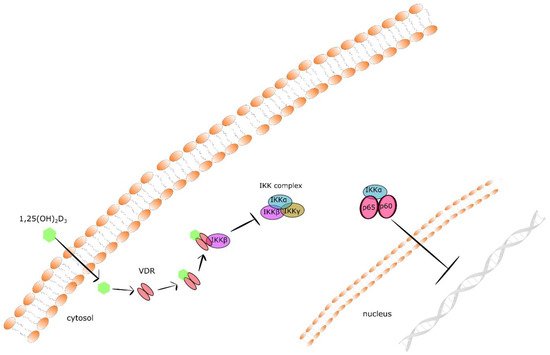
Taking into consideration that CYP24A1 degrades both calcidiol and calcitriol, its expression might be upregulated by cancer cells and lead to reduced local concentrations of 1,25(OH)
2D
3 reported by Albertson et al., who found amplified CYP24A1 in breast cancer [
67]. The increased expression of CYP24A1 was demonstrated to be correlated with the advanced stages of prostate, colon, lung and breast cancers, stimulating resistance to vitamin D-mediated therapy [
57,
61,
68,
69,
70,
71,
72]. The overexpression of CYP24A1 has been also documented in numerous other types of cancer, including cervical, ovarian, squamous cell and basal cell carcinoma [
73,
74]. Moreover, CYP24A1 up-expression is related to poor prognosis in colon, lung and esophageal cancer [
68,
75,
76] The oncogenic role of CYP24A1 is supported by results of studies presented that the suppression of CYP24A1 inhibited tumor growth and strengthened antitumorigenic effects of 1,25(OH)
2D
3 in breast and lung cancers [
77,
78,
79]. However, opposite data have been also published for prostate cancer [
70,
80] and a negative correlation between expression of CYP24A1 and tumor progression has been observed in melanoma [
81].
It was proposed that increased CYP24A1 expression observed in cancer cells is probably mediated via activation of VDR, because both activity and expression of VDR are downregulated in most types of cancer. Moreover, the overexpression of CYP24A1 in numerous cancer cells may not be a result of normal physiological processes mediated by calcitriol–VDR-dependent mechanisms. Firstly, it has been shown that overexpression of CYP24A1 in breast cancer is related to the amplification of chromosomal locus 20q13.2–20q13.3 comprising the CYP24A1 gene, that has been also found in other types of cancer, including colon malignancies [
67,
82]. The amplification of CYP24A1 was identified only in malignant, but not benign colon tumors, thus these observations suggest that CYP24A1 overexpression and inactivation of calcitriol may be a key feature of tumor cells [
83]. Secondly, epigenetic modifications, namely DNA methylation of the promoter region leads to modification of
CYP24A1 expression in cancer cells. It has been reported that
CYP24A1 expression is negatively correlated with the methylation of the
CYP24A1 promoter in prostate and lung cancer, in vivo and in vitro [
75,
83] Thirdly, the suppression of DNA methyltransferase (DNMT) or histone deacetylase (HDAC) elevated
CYP24A1 expression in colon and lung cancer. Fourthly, post-transcriptional regulation via microRNAs is related to the
CYP24A1 overexpression in cancer. The expression of
CYP24A1 has been found to be inversely correlated to the expression of miR-125b in breast cancer [
84], suggesting that decreased levels of miR-125b may be responsible for
CYP24A1 overexpression in cancer. The results of recent study also showed that the miR-17 to -92 cluster also control
CYP24A1 expression in lung cancer cells [
85]. It is also known that the serine/threonine protein kinase casein kinase 2 (CK2) signaling pathway stimulates overexpression of
CYP24A1 in prostate cancers. CK2 is involved in the regulation of
CYP24A1 expression by 1,25(OH)
2D
3 and the CK2 inhibitor enhances 1,25(OH)
2D
3-mediated antitumor effect [
86]. Moreover,
CK2 overexpression has been shown in numerous cancers, including prostate, pancreatic, breast, colon and rectum, lung and bronchus cancer [
87]. It should be underlined that
CK2 overexpression was found to be related to poor clinical outcomes [
88].