Heart failure (HF) is one of the major causes of morbidity and mortality worldwide and represents an escalating problem for healthcare system. Therefore, it would be of utmost importance to identify asymptomatic individuals with left ventricular dysfunction before the onset of symptoms. Furthermore, special attention should be focused on individuals who are already classified as NIHA I and "apparently healed" patients, who have been diagnosed with HF and whose clinical condition is stable thanks to therapy. These patients usually suffer from a worsening of their condition over time, and therefore recognizing these changes at the onset would be a great achievement.
- asymptomatic heart failure
- apparently healed patients
- cardiovascular diseases
- biomarkers
- diagnostic
1. Introduction
Despite the progress made in the field of cardiology in terms of treatment and personalized therapy, heart failure (HF) remains one of the leading causes of morbidity and mortality [1]. Unfortunately, the diagnosis of HF in asymptomatic subjects is challenging and therefore delayed until overt HF [2].
2. Current and Emerging Protein Biomarkers
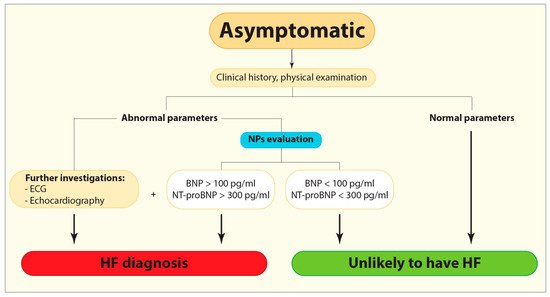
Figure 1. Flow diagram demonstrating the current diagnostic algorithm for HF identification. BNP, B-type or brain natriuretic peptide; ECG, Electrocardiogram; HF, Heart failure; NPs, Natriuretic peptides; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Cardiac troponin is the gold biomarker for diagnosis of acute myocardial infarction (AMI) and its elevated levels are present in patients with acute and chronic HF as well, indicating the ongoing myocardial damage [9][11]. Troponin levels may be elevated regardless of the ischemic etiology of myocardial injury due to different causes such as LV hypertrophy, hypertension, diabetes mellitus, metabolic syndromes, hypercholesterolemia, and genetics, so its detection in the general population has aroused interest in this marker as an indicator of myocardial injury in asymptomatic individuals [12][13][14]. However, defining the cut-offs among asymptomatic individuals might be challenging since different factors significantly affect troponin levels such as age, gender, body mass index, systolic pressure, diabetes mellitus, severe pulmonary infections and renal failure [12][13][15][16].
Emerging biomarkers such as soluble suppression of tumorigenicity 2 (sST2), galectin-3 (Gal-3) and ghrelin reflect different pathophysiological processes closely associated with fibrosis [12]. Even though the diagnostic potential of these relatively new biomarkers is still lower compared to NPs, their prognostic power proved to be very useful during patient’s follow-up. The American College of Cardiology guidelines, besides a strong recommendation of the use of NPs, has already suggested a multimarker approach since these emerging biomarkers could provide additional diagnostic information to the traditional biomarkers, as well as help regarding risk stratification [8].
Elevated levels of Gal-3 have been detected in patients with LV remodeling and diastolic dysfunction [20]. Huttin et al. demonstrated a significant association between Gal-3 and fibrosis at a very early stage of cardiac changes [21]. However, the use of Gal-3 as a biomarker for asymptomatic LV dysfunction is still debated, mainly due to its low specificity and the fact that its concentration levels could be altered due to inflammation and fibrotic processes in organs other than the heart [22].
Finally, ghrelin, primarily recognized as a gastric peptide, is expressed in the heart, although at a much lower level than in the gastrointestinal tract [23][24]. Given its cardioprotective role against apoptosis and myocardial fibrosis [24], ghrelin and its receptor have become the subject of intensive studies. It has been noted that the interaction between ghrelin and its receptor differs between the early and late phases of HF [24] and that changes in the ghrelin myocardial axis could be detectable even before overt changes in LV function [23]. In our previous study, we observed lower levels of ghrelin in patients with DCM in comparison with healthy controls [24]. Interestingly, among DCM cohort, early diagnosed patients had higher ghrelin levels than patients with a longer duration of the disease [24].
3. Promising New Biomarkers
Promising biomarkers that pave their pathways in the cardiovascular fields are microRNA (miRNA), long non-coding RNAs (lncRNA) and exosomes. These molecules are able to give us an insight into various processes involved in HF development such as myocyte loss, hypertrophy, fibrosis and changes in the extracellular matrix that are involved in cardiac remodeling. miRNAs involved in coronary artery disease (CAD) (e.g., miR-624, miR-340, miR-15-5p, miR-21-5p, miR-210-5p, miR-29b-3p, miR-7-5p, miR-99a-5p) [26][27], diabetes (e.g., miR-21) [28], and hyperlipidemia (e.g., miR-122, miR-370) [29][30] could be used as biomarkers since these conditions increase the likelihood of HF development overt time. In the same context, being associated with a higher incidence of diabetes, antisense lncRNA such as CTBP1-AS2 and VIM-AS1 have the potential as prognostic and diagnostic markers as well[31][32].
β secretase-1 antisense (BACE1-AS) lncRNA and exosomes cargo, consisting of exo-miRNA-192, exo-miRNA-194, and exo-miRNA-134a might be indicators of cardiomyocyte death[33], whereas CTBP1-AS2 and VIM-AS1 mediate cardiomyocyte hypertrophy and fibrosis respectively [34][35]. In addition, exosome cargo consisting of exo-miRNA-21-3p, exo-miRNA-132, and exo-miRNA-200 [36] and non-coding RNAs such as miR-1, miR-133a indicate the early phases of hypertrophy and could be very helpful in its timely identification. [37].
4. Conclusion
Early recognition of asymptomatic individuals prior to overt HF development would reduce morbidity and mortality associated with the disease. Traditional and emerging biomarkers are reserved for the symptomatic phase, therefore there is a need for further studies regarding their practical use in asymptomatic settings. Non-coding RNA and exosomes cargo, by the same token, may provide an insight into undergoing processes that could lead to HF over time and therefore could be very useful as prognostic biomarkers. Given the fact that different biomarkers are involved in multiple harmful pathophysiological processes associated with HF, the multimarker approach might enable the early identification of patients at risk of HF.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094937
References
- Aleksova, A.; Sabbadini, G.; Merlo, M.; Pinamonti, B.; Barbati, G.; Zecchin, M.; Bussani, R.; Silvestri, F.; Iorio, A.M.; Stolfo, D.; et al. Natural history of dilated cardiomyopathy: From asymptomatic left ventricular dysfunction to heart failure—A subgroup analysis from the Trieste Cardiomyopathy Registry. J. Cardiovasc. Med. (Hagerstown) 2009, 10, 699–705.
- Chien, S.-Y.; Chuang, M.-C.; Chen, I. Why people do not attend health screenings: Factors that influence willingness to participate in health screenings for chronic diseases. Int. J. Environ. Res. Public Health 2020, 17, 3495.
- Halliday, B.P.; Wassall, R.; Lota, A.S.; Khalique, Z.; Gregson, J.; Newsome, S.; Jackson, R.; Rahneva, T.; Wage, R.; Smith, G.; et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. Lancet 2019, 393, 61–73.
- Merlo, M.; Stolfo, D.; Anzini, M.; Negri, F.; Pinamonti, B.; Barbati, G.; Ramani, F.; Lenarda, A.D.; Sinagra, G. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: Does real healing exist? J. Am. Heart Assoc. 2015, 4, e001504.
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018, 6, 179–186.
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017 Aug 8;70(6):776-803. Epub 2017 Apr 28. PMID: 28461007.
- Gualandro, D.M.; Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Puelacher, C.; Muller, C. Biomarkers in cardiovascular medicine: Towards precision medicine. Swiss Med. Wkly. 2019, 149, w20125.
- Sara, J.D.; Toya, T.; Taher, R.; Lerman, A.; Gersh, B.; Anavekar, N.S. Asymptomatic Left Ventricle Systolic Dysfunction. Eur. Cardiol. 2020, 15, e13.
- Latini, R.; Masson, S.; Anand, I.S.; Missov, E.; Carlson, M.; Vago, T.; Angelici, L.; Barlera, S.; Parrinello, G.; Maggioni, A.P.; et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007, 116, 1242–1249.
- Shemisa, K.; Bhatt, A.; Cheeran, D.; Neeland, I.J. Novel Biomarkers of Subclinical Cardiac Dysfunction in the General Population. Curr. Heart Fail. Rep. 2017, 14, 301–310.
- Farmakis, D.; Mueller, C.; Apple, F.S. High-sensitivity cardiac troponin assays for cardiovascular risk stratification in the general population. Eur. Heart J. 2020, 41, 4050–4056.
- Welsh, P.; Preiss, D.; Hayward, C.; Shah, A.S.V.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Padmanabhan, S.; Welsh, C.; et al. Cardiac Troponin T and Troponin I in the General Population. Circulation 2019, 139, 2754–2764.
- Drazner, M.H.; Rame, J.E.; Marino, E.K.; Gottdiener, J.S.; Kitzman, D.W.; Gardin, J.M.; Manolio, T.A.; Dries, D.L.; Siscovick, D.S. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2004, 43, 2207–2215.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167(3):276-81.
- Wang, T.J.; Wollert, K.C.; Larson, M.G.; Coglianese, E.; McCabe, E.L.; Cheng, S.; Ho, J.E.; Fradley, M.G.; Ghorbani, A.; Xanthakis, V.; et al. Prognostic utility of novel biomarkers of cardiovascular stress: The Framingham Heart Study. Circulation 2012, 126, 1596–1604.
- van Vark, L.C.; Lesman-Leegte, I.; Baart, S.J.; Postmus, D.; Pinto, Y.M.; Orsel, J.G.; Westenbrink, B.D.; Brunner-la Rocca, H.P.; van Miltenburg, A.J.M.; Boersma, E.; et al. Prognostic Value of Serial ST2 Measurements in Patients With Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 2378–2388.
- Aleksova, A.; Paldino, A.; Beltrami, A.P.; Padoan, L.; Iacoviello, M.; Sinagra, G.; Emdin, M.; Maisel, A.S. Cardiac Biomarkers in the Emergency Department: The Role of Soluble ST2 (sST2) in Acute Heart Failure and Acute Coronary Syndrome—There is Meat on the Bone. J. Clin. Med. 2019, 8, 270.
- de Boer, R.A.; Yu, L.; van Veldhuisen, D.J. Galectin-3 in cardiac remodeling and heart failure. Curr. Heart Fail. Rep. 2010, 7, 1–8.
- Huttin O, Kobayashi M, Ferreira JP, Coiro S, Bozec E, Selton-Suty C, Filipetti L, Lamiral Z, Rossignol P, Zannad F, Girerd N. Circulating multimarker approach to identify patients with preclinical left ventricular remodelling and/or diastolic dysfunction. ESC Heart Fail. 2021 Apr;8(2):1700-1705.
- Hara, A.; Niwa, M.; Kanayama, T.; Noguchi, K.; Niwa, A.; Matsuo, M.; Kuroda, T.; Hatano, Y.; Okada, H.; Tomita, H. Galectin-3: A Potential Prognostic and Diagnostic Marker for Heart Disease and Detection of Early Stage Pathology. Biomolecules 2020, 10, 1277.
- Sullivan, R.; Randhawa, V.K.; Lalonde, T.; Yu, T.; Kiaii, B.; Luyt, L.; Wisenberg, G.; Dhanvantari, S. Regional Differences in the Ghrelin-Growth Hormone Secretagogue Receptor Signalling System in Human Heart Disease. CJC Open 2021, 3, 182–194.
- Aleksova, A.; Beltrami, A.P.; Bevilacqua, E.; Padoan, L.; Santon, D.; Biondi, F.; Barbati, G.; Stenner, E.; Gortan Cappellari, G.; Barazzoni, R.; et al. Ghrelin Derangements in Idiopathic Dilated Cardiomyopathy: Impact of Myocardial Disease Duration and Left Ventricular Ejection Fraction. J. Clin. Med. 2019, 8, 1152.
- Howlett, J.G.; Sharma, N.; Alemayehu, W.G.; Dyck, J.R.B.; Anderson, T.; Fine, N.; Becker, H.; White, J.A.; Paterson, D.I.; Thompson, R.B.; et al. Circulating troponin and further left ventricular ejection fraction improvement in patients with previously recovered left ventricular ejection fraction. ESC Heart Fail. 2020, 7, 2725–2733.
- Sondermeijer, B.M.; Bakker, A.; Halliani, A.; de Ronde, M.W.; Marquart, A.A.; Tijsen, A.J.; Mulders, T.A.; Kok, M.G.; Battjes, S.; Maiwald, S.; et al. Platelets in patients with premature coronary artery disease exhibit upregulation of miRNA340* and miRNA624*. PLoS ONE 2011, 6, e25946.
- Patterson, A.J.; Song, M.A.; Choe, D.; Xiao, D.; Foster, G.; Zhang, L. Early Detection of Coronary Artery Disease by Micro-RNA Analysis in Asymptomatic Patients Stratified by Coronary CT Angiography. Diagnostics 2020, 10, 875
- Tao, L.; Huang, X.; Xu, M.; Qin, Z.; Zhang, F.; Hua, F.; Jiang, X.; Wang, Y. Value of circulating miRNA-21 in the diagnosis of subclinical diabetic cardiomyopathy. Mol. Cell. Endocrinol. 2020, 518, 110944.
- Omran, A.; Elimam, D.; He, F.; Peng, J.; Yin, F. Potential role of blood microRNAs as non-invasive biomarkers for early detection of asymptomatic coronary atherosclerosis in obese children with metabolic syndrome. Med. Hypotheses 2012, 79, 889–893.
- Gao, W.; He, H.W.; Wang, Z.M.; Zhao, H.; Lian, X.Q.; Wang, Y.S.; Zhu, J.; Yan, J.J.; Zhang, D.G.; Yang, Z.J.; et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012, 11, 55.
- Luo, X.; He, S.; Hu, Y.; Liu, J.; Chen, X. Sp1-induced LncRNA CTBP1-AS2 is a novel regulator in cardiomyocyte hypertrophy by interacting with FUS to stabilize TLR4. Cardiovasc. Pathol. 2019, 42, 21–29.
- Zhao, X.; Jiang, X.; Liu, Z.; Zhou, M.; Zhang, J.; Wang, X.; Li, X. Long Noncoding RNA VIM Antisense RNA 1 (VIM-AS1) Plays an Important Role in Development of Preeclampsia by Regulation of Epithelial Mesenchymal Transition. Med. Sci. Monit. 2019, 25, 8306–8314.
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.; Zornoff, L.A. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62–69.
- Luo, X.; He, S.; Hu, Y.; Liu, J.; Chen, X. Sp1-induced LncRNA CTBP1-AS2 is a novel regulator in cardiomyocyte hypertrophy by interacting with FUS to stabilize TLR4. Cardiovasc. Pathol. 2019, 42, 21–29
- Zhao, X.; Jiang, X.; Liu, Z.; Zhou, M.; Zhang, J.; Wang, X.; Li, X. Long Noncoding RNA VIM Antisense RNA 1 (VIM-AS1) Plays an Important Role in Development of Preeclampsia by Regulation of Epithelial Mesenchymal Transition. Med. Sci. Monit. 2019, 25, 8306–8314
- Xu, J.Y.; Chen, G.H.; Yang, Y.J. Exosomes: A Rising Star in Falling Hearts. Front. Physiol. 2017, 8, 494.
- Wehbe, N.; Nasser, S.A.; Pintus, G.; Badran, A.; Eid, A.H.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714.
