You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Skeletal muscle has an outstanding capacity for regeneration in response to injuries, but there are disorders in which this process is seriously impaired, such as sarcopenia. Pharmacological treatments to restore muscle trophism are not available, therefore, the identification of suitable therapeutic targets that could be useful for the treatment of skeletal reduced myogenesis is highly desirable.
- skeletal muscle
- satellite cells
- myogenesis
- calcium-sensing receptor
- G protein-coupled receptors
1. Introduction
Skeletal muscle tissue has an inherent capacity for regeneration in response to minor injuries. However, there are circumstances that can cause irreversible loss of muscle mass and function, for example, severe trauma, tumor ablation, and different types of congenital or acquired muscle diseases, such as muscular dystrophies and sarcopenia [1][2][3][4][5][6].
Pathological situations, in which skeletal muscle regeneration is seriously impaired, represent conditions that affect motor unit function with significant disabilities and reduced quality of life (QoL) in people who are affected. Pharmacological treatments are not available for these muscle disorders. Hence, there is an unmet need for pharmacological strategies for the treatment of skeletal muscle degenerative disorders.
Postnatal skeletal muscle stem cells, called satellite cells (SCs), are responsible for skeletal muscle regeneration during the lifespan. Several studies report that some myopathies, such as sarcopenia and muscular dystrophies, are related to a failure of SCs to enter into the myogenic program [7][8]. As SCs are committed myogenic precursors, and possess the capability of self-renewal, a unique feature of stem cells, they play a central role in the search for therapies for skeletal muscle disorders [9]. The identification of new therapies, and the testing of candidate drugs for their ability to improve muscle regeneration, require appropriate in vitro cell-based screening assays to select compounds or new targets for further development. The use of human skeletal muscle-derived cells is an essential and extremely powerful cell-based model system that may support and predict from in vivo results the in vitro effects obtained in the laboratory, which could later be useful for finding therapeutic drugs targeted for skeletal muscle degenerative myopathies [10].
In the last decade, it has become possible to isolate and culture a population of SCs [11][12][13][14]. While the use of primary cultures of SCs also has some inherent drawbacks, mainly relating to the fact that they are generally slow growing and can undergo a limited number of divisions [15], SCs represent an important in vitro model to understand skeletal muscle physiology and pathology.
Calcium ion (Ca2+) is a ubiquitous intracellular signal that regulates a myriad of cellular processes, as it is one of the most specific and selective messengers in nature. It is involved in multiple signaling cascades critical for cell survival, growth, differentiation, and death [16][17]. It is essential for muscle function, and the tight coupling of muscle excitation and contraction by Ca2+ dynamics has been well established. Voltage-gated Ca2+ channels are key transducers which are activated by membrane depolarization and mediate Ca2+ influx in response to an action potential. Ca2+ entering the cell through voltage-gated Ca2+ channels serves as the second messenger of electrical signaling that initiates many different cellular events [18]. Moreover, many studies have shown the importance of Ca2+ for skeletal muscle development, maintenance, and regeneration [19][20][21].
The calcium-sensing receptor (CaSR) is a key systemic regulator of calcium homeostasis [22], acting as a modulator of extracellular Ca2+ concentrations, and it has been proven to be an important molecule in physiology and pathology [16]. It belongs to the class C G protein-coupled receptors (GPCRs), also known as 7-transmembrane domain receptors, a large protein family of receptors that sense molecules outside the cell and activate internal signal transduction pathways and, ultimately, cellular responses [23]. The CaSR is primarily expressed within parathyroid glands where it regulates parathyroid hormone (PTH) secretion, which in turn controls calcium homeostasis, by acting upon kidney, bone, and intestine to maintain Ca2+ concentrations within the physiological range (1.1–1.3 mM) [22]. Initially, studies of the CaSR were focused on calciotropic tissues, such as parathyroid, kidney, and bone, which have obvious roles in maintaining calcium homeostasis. Now, it has become clear that the CaSR is expressed in many other cells and organs (such as neurons, lungs, skin, placenta, breast, and blood vessels), without any evident role in maintaining calcium homeostasis [17]. Several studies have observed that abnormal CaSR expression and function are implicated not only in calciotropic disorders, such as hyper- and hypoparathyroidism, but also in diseases linked to non-calciotropic systems, such as of the nervous, reproductive, and respiratory systems, and even in diseases, such as chronic inflammation and cancer [16][17][22].
Drugs targeting CaSR in related pathologies are already available [24]. Moreover, several recent scientific studies have observed a role of CaSR specifically in the physiological differentiation of cells, such as keratinocytes, cardiomyocytes, and neurons, and in smooth muscle contractility and proliferation [25][26][27][28][29].
Despite the importance of this receptor, the expression and function of the CaSR in skeletal muscle has not been explored. Therefore, the aim of our study was to investigate the presence of CaSR in human skeletal muscle and its possible role in skeletal muscle myogenic differentiation, in order to find new possible therapeutic targets for skeletal muscle degenerative diseases.
2. Expression and Function of Calcium-Sensing Receptor in Human Skeletal Muscle
2.1. Isolation of Primary Culture of Human Satellite Cells (hSCs) and Characterization of Established hSC Lines
Satellite cells (hSCs) were isolated from human skeletal muscle biopsies, as described recently [14], and subcultured in Matrigel-coated plates (Figure 1A,B).
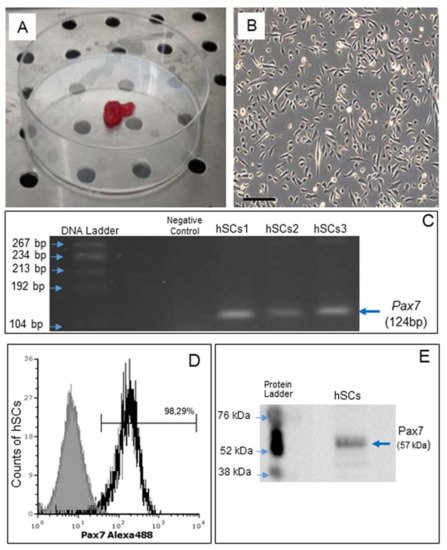
Figure 1. Human skeletal muscle biopsy (A) and primary cell culture of hSCs visualized by phase contrast microscopy, original magnification: 10×, scale bar 200 µm (B). RT-PCR analysis of Pax7 gene expression in hSC lines (hSCs1, hSCs2, hSCs3), passage 1. Negative control: PCR without cDNA template (C). Representative flow cytometry analysis of Pax7 protein expression in hSC lines, area in black boundaries represents the hSCs stained with primary anti-Pax7 antibody whereas the area under gray shading represents the hSCs only stained with secondary antibody (D). Representative Western blot analysis of Pax7, confirming the presence of Pax7 protein in hSCs (E).
Gene expression of Pax7, a nuclear transcriptional factor and the main marker of SCs [30], was confirmed by RT-PCR (Figure 1C). By flow cytometry, Pax7 protein expression was demonstrated in 98.29% of all cells (Figure 1D) and confirmed by Western blot analysis (Figure 1E).
2.2. In Vitro Myogenic Differentiation of hSCs
Confluent hSCs, at 70–80% density in the plate, were differentiated toward the myogenic phenotype using an appropriate myogenic differentiation medium for 9 days. During this period, the activated cells align (myoblasts), then subsequently fuse with each other and, finally, differentiate into multinucleated myofibers. Phase contrast microscopy revealed the presence of multinucleated elongated cells, containing from three to more than eight nuclei, related to myotubes (Figure 2A).
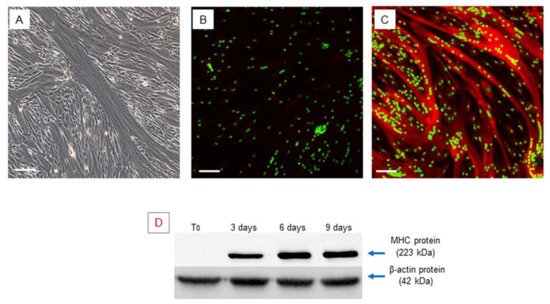
Figure 2. Myogenic differentiation: multinucleated cells (myotubes) observed after 9 days of myogenic induction, visualized by phase contrast microscopy, original magnification: 20×, scale bar 50 µm (A). Immunofluorescence staining of MHC in the obtained multinucleated cells. The obtained myotubes showed a high positivity of MHC protein, (A) negative control, myotubes stained with secondary antibody only, (B) myotubes stained with anti-MHC antibody, LSCM: red for MHC and green for nuclei, original magnification: 10×, scale bar 200 µm. (B,C). Western blot analysis of MHC during myogenic differentiation. The analysis showed an increase in the MHC gene during myogenic differentiation. Experiment was carried out in triplicate and is representative of the three established hSC lines (D).
Myogenic differentiation was confirmed by expression of the myosin heavy chain (MHC), which is the most important terminal myogenic differentiation marker protein [31]. After 9 days of myogenic induction, the multinucleated cells exhibited high MHC expression, as confirmed by immunofluorescence microscopy (Figure 2B,C).
The myogenic phenotype was also confirmed by RT-PCR analysis to evaluate the expression of the myogenic regulatory factor genes Myf5, MyoD1, Myogenin, and the terminal differentiation marker MHC. We observed that these genes were expressed at the same time during the last period (9 days) of myogenic differentiation when myotubes were formed, while during the first phase (T0) of the myogenic differentiation, only Myf5 and MyoD1 were expressed, in accordance with the literature, while Myogenin and MHC were completely absent (Table 1). The earlier detection of MyoD1 that we found in the primary cultured hSCs is due to the fact that after collecting biopsies, the surgical procedure per se is a form of injury that initiates muscle regeneration, which leads to the activation of satellite cells and to their commitment to myogenic differentiation.
Table 1. RT-PCR analysis of myogenic differentiation marker genes. RT-PCR analysis showed the different expression of Myf5, MyoD1, Myogenin, and of MHC, analyzed over time during myogenic differentiation of three primary hSC lines (hSCs1, hSCs2, hSCs3).
| MRFs Genes | T0 HSCs1 |
T0 HSCs2 |
T0 HSCs3 |
7 Days hSCs1 |
7 Days hSCs2 |
7 Days hSCs3 |
|---|---|---|---|---|---|---|
| Myf5 | + | + | + | + | + | + |
| MyoD1 | + | + | + | + | + | + |
| Myogenin | -- | -- | -- | + | + | + |
| MHC | -- | -- | -- | + | + | + |
MHC protein expression was also evaluated by Western blot analysis at different time points (T0–3–6–9 days) of myogenic differentiation and revealed a gradual increase in MHC over time during myogenic differentiation progression (Figure 2D), validating the suitability of the in vitro myogenesis model.
2.3. Analysis of CaSR Gene Expression in Human Skeletal Muscle Tissues (hSMts) and in the Derived Human Satellite Cell (hSC) Lines and during In Vitro Myogenesis
We performed qualitative RT-PCR using different pairs of primers that target exons 2/3, 4/5, and 6/7 of the human CaSR gene (Table 2). CaSR mRNA expression was neither detected in human skeletal muscle tissues (hSMt1, hSMt2, hSMt3), nor in the respective established human satellite cell lines (hSCs1, hSCs2, hSCs3), using any of the primer settings (Figure 3A–C). A primary cell culture of human adenoma parathyroid cells (PTcs) was used as a positive control. On the other hand, human embryonic HEK293, used as a negative control, did not show CaSR expression, as expected [32]. Housekeeping gene β-actin was used to verify the appropriate quality of the assayed samples (Figure 3D).
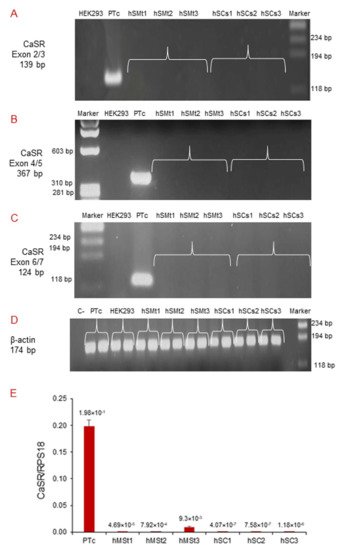
Figure 3. Molecular analysis of CaSR gene expression in hSMts and established hSC lines. The analysis in RT-PCR showed the expression of CaSR gene using different pairs of primers that fall into: (A) exons 2/3; (B) exons 4/5; (C) exons 6/7. The gene was not detected in any of the samples. The analysis shows the presence of CaSR only in human PTcs that were used as a positive control. HEK293 were used as a negative control. Housekeeping β-actin gene analysis of the assayed samples (D). Real-time qPCR analysis of CaSR gene in hSMts and in hSC lines. The analysis showed a very low expression of CaSR gene in hSMts and in hSC lines. PTcs were used as positive control (E).
Table 2. List of the human gene-specific primers used in the analysis.
| Name of Gene | Primer Sequence | Tm (°C) | Amplicon Size (bp) |
|---|---|---|---|
| β-actin | Forward 5′-AGCCTCGCCTTTGCCGA-3′ | 60 °C | 174 |
| Reverse 5′-CTGGTGCCTGGGGCG-3′ | |||
| Pax7 | Forward 5′-GGTACCGAGAATGATGCGG-3′ | 55 °C | 124 |
| Reverse 5′-CCCATTGATGAAGACCCCTC-3′ | |||
| Myf5 | Forward 5′-ATGCCATCCGCTACATCG-3′ | 55 °C | 145 |
| Reverse 5′-ACAGGACTGTTACATTCGGC-3′ | |||
| MyoD1 | Forward 5′-GACGTGCCTTCTGAGTCG-3′ | 55 °C | 148 |
| Reverse 5′-CTCAGAGCACCTGGTATATCG-3′ | |||
| Myogenin | Forward 5′-AGCGAATGCAGCTCTCAC-3′ | 55 °C | 150 |
| Reverse 5′-TGTGATGCTGTCCACGATG-3′ | |||
| MHC | Forward 5′-GAGTCCTTTGTGAAAGCAACAG-3′ | 55 °C | 143 |
| Reverse 5′-GCCATGTCCTCGATCTTGTC-3′ | |||
| CaSR exons 2/3 | Forward 5′-GATCAAGATCTCAAATCAAG-3′ | 57 °C | 139 |
| Reverse 5′-CCAGCGTCAAGTTGGGAAGA-3′ | |||
| CaSR exons 4/5 | Forward 5′-CTGAGAGGTCACGAAGAAAGTG-3′ | 58 °C | 367 |
| Reverse 5′-GGTGCCAGTTGATGATGGAATA-3′ | |||
| CaSR exons 6/7 | Forward 5′-CTGCTGCTTTGAGTGTGTGG-3′ | 60 °C | 124 |
| Reverse 5′-CTTGGCAATGCAGGAGGTGT-3′ |
Tm: melting temperature; bp: base pairs of amplicon.
Real-time qPCR detected very low CaSR expression levels in hSMts and in the established hSC lines, but since the quantity was extremely small in all of the assayed samples, it was not possible to sequence the amplicons to confirm the detected mRNA products (Figure 3E).
Subsequently, since during in vitro myogenesis satellite cells mature and gradually express receptors [14], we analyzed CaSR gene expression by RT-PCR at T0 and after 9 days of myogenic differentiation, using all three different pairs of primers previously utilized. The analyses showed no CaSR gene expression at these experimental time points, demonstrating the absence of CaSR gene during the in vitro myogenesis of the three primary hSC lines. CaSR gene expression was demonstrated in PTcs, while it was absent from HEK293 cells. Housekeeping gene β-actin was used in order to verify the appropriate quality of the assayed samples (Figure 4A–D). Additionally, real-time qPCR was also performed to evaluate the possible expression of CaSR during myogenesis. We evaluated CaSR gene expression at T0, 3, 6, and 9 days of myogenic differentiation of the three primary hSC lines. Data verify the absence of CaSR during myogenic induction, confirming the data reported in Figure 3, and assuming that this gene is not present during the differentiation process of the hSCs (Figure 4E,F).
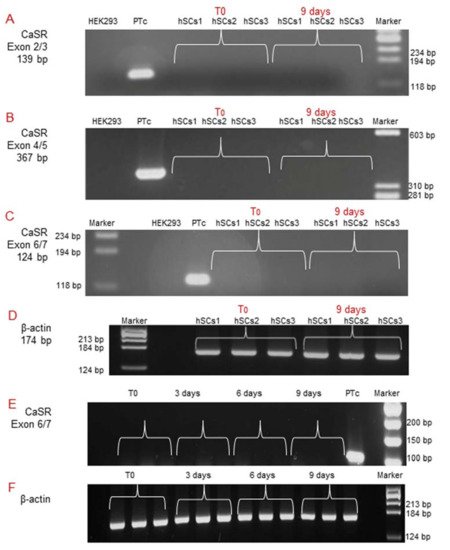
Figure 4. Molecular analysis of CaSR gene expression during in vitro myogenesis of the hSC lines. The analysis showed the absence of the CaSR gene at T0 and at 9 days of myogenic differentiation of 3 primary hSC lines using different pairs of primers that fall into: (A) exons 2/3; (B) exons 4/5; (C) exons 6/7. The primary cultured human PTcs were used as a positive control and HEK293 cells were used as a negative control. Housekeeping β-actin gene analysis of the assayed samples (D). Real-time qPCR analysis of CaSR gene at different time points T0, 3, 6, 9 days of myogenesis of the established hSC lines. The analysis showed no detection of expression of CaSR gene in hSC lines. Human primary cultured parathyroid cells (PTcs) were used as positive control (E). Negative control: PCR without template cDNA in β-actin gene analysis (F). Experiments were carried out in triplicate and are representative of the three established hSC lines.
2.4. CaSR Protein Expression Analysis in Human Skeletal Muscle Tissues (hSMts), in hSCs, and during In Vitro Myogenesis
After analyzing the CaSR gene expression, our study was focused on the evaluation of a possible expression of CaSR protein in hSMt sections, in the established hSC lines, and during myogenic differentiation. We performed: (a) Western blot analysis, (b) immunofluorescence staining, and (c) flow cytometry, in order to assess the presence of the protein CaSR.
CaSR protein expression in primary hSC lines was investigated by Western blot analysis, by which it is possible to evaluate the size of the CaSR protein either in monomers of 140 and 160 kDa (in reducing condition with β-mercaptoethanol or 50 mM DTT, dithiothreitol, disulfide bond breaker) or in dimers of 240 kDa (non-reducing condition) forms.
The analysis, in standard reducing conditions, was performed in primary hSC lines at different time points: T0, 3, 6, and 9 days of myogenic differentiation. The specificity of the antibody was demonstrated using HEK293 cells, stably transfected with human CaSR (HEKCaSR), and used as a positive control. The obtained results showed very faint bands of about 140 kDa and 160 kDa (size of CaSR monomers) in the basal hSC lines and during myogenic differentiation (Figure 5A).
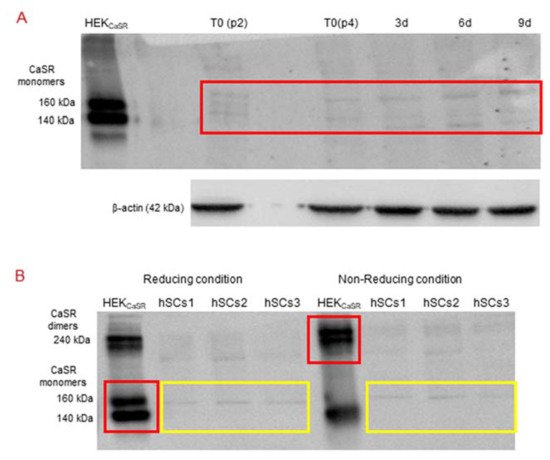
Figure 5. (A) Western Blot (standard reducing condition) for CaSR protein in hSC lines and during myogenic differentiation. The results show the presence of faint bands of about 160 kDa and 140 kDa in hSC lines and at different time points (T0, 3, 6, 9 days of myogenic differentiation) of primary hSC lines. HEKCaSR were used as positive control. Experiments were carried out in triplicate and are representative of the three established hSC lines. (B) Western blot analysis of CaSR in reducing and non-reducing conditions. The analysis for CaSR protein in three hSC lines showed that the observed bands are not CaSR as they did not shift to dimer form at 240 kDa in non-reducing conditions. HEKCaSR were used as positive control. The red and yellow boxes surround the analyzed bands.
Since we had not observed the CaSR gene expression in the established hSC lines and during myogenic differentiation, but did observe faint bands in Western blot analyses with similar molecular weight of CaSR monomer bands in the same samples, we analyzed the samples under reducing and non-reducing conditions by Western blot.
The obtained results showed that the monomer CaSR bands, observed in the positive control HEKCaSR in a standard reducing condition, shifted to the CaSR dimer band at 240 kDa in non-reducing conditions. On the other hand, the monomer bands in the hSC lines, obtained in standard reducing conditions, did not shift to the dimer band at 240 kDa in the non-reducing condition (Figure 5B).
Then, immunofluorescence analysis of CaSR protein was performed. The hSMt sections (Figure 6A–C) show how human skeletal muscle appears in phase contrast microscopy, with the longitudinal view of the skeletal muscle fibers.
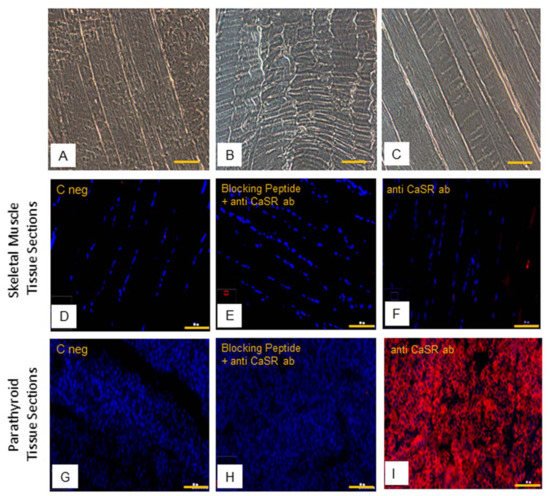
Figure 6. Human skeletal muscle tissue (hSMt) sections; observations in phase contrast microscopy, original. Magnification: 10×, scale bar 200 µm (A–C). Immunostaining of CaSR protein. Analysis of CaSR protein in human skeletal muscle tissue sections: (D) negative control only with secondary antibody, (E) control with anti-CaSR antibody absorbed with blocking peptide, and (F) with primary anti-CaSR antibody. Analysis of CaSR protein in human parathyroid tissue sections used as positive control: (G) negative control with only secondary antibody, (H) control with anti-CaSR antibody absorbed with blocking peptide, and (I) with primary anti-CaSR antibody. Fluorescent microscopy in conventional colors: red for CaSR and blue for nuclei, original magnification: 10×, scale bar: 200 µm. Experiment was carried out in triplicate (in hSMt sections of three different humans).
The results of immunofluorescence analysis revealed no staining of CaSR in hSMt sections (Figure 6D,F). In comparison to that, positive detection of CaSR expression in human parathyroid tissue sections validated our method of analysis (Figure 6G,I). The specificity of the anti-CaSR antibody was verified by one extra internal negative control in the assay, where the antibody binding was blocked by pre-absorption with the blocking peptide [33][34]. The results demonstrate the absence of CaSR protein expression in hSMts (Figure 6E,H).
The subsequent immunostaining performed in hSC lines also showed no detection of CaSR protein in satellite cells (Figure 7A,B). Human PTcs were used as a positive control, to validate the method and anti-CaSR antibody (Figure 7C,D).
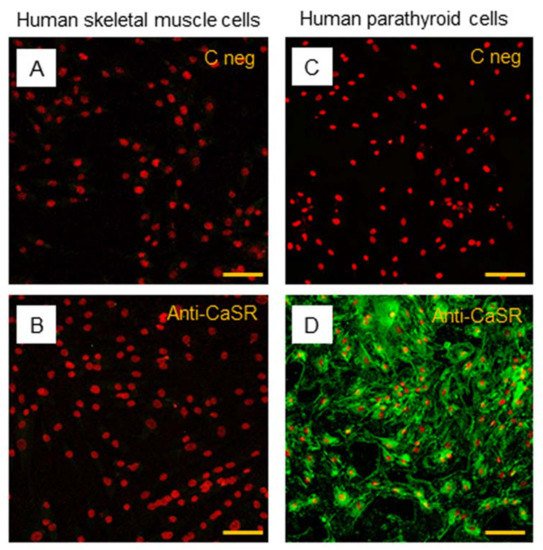
Figure 7. Immunostaining of CaSR in hSC lines. The analysis of CaSR protein in hSC lines: (A) negative control with only secondary antibody and (B) with primary anti-CaSR antibody. Results are representative of experiments carried out in three established hSC lines. The analysis of CaSR protein was also performed in human parathyroid cells, used as positive control, (C) negative control with only secondary antibody, and (D) with primary anti-CaSR antibody. LSCM conventional colors: green for CaSR protein and red for nuclei, original magnification: 20×, scale bar: 50 µm.
In addition to immunostaining, we performed flow cytometry using two different dilutions (1:500 and 1:100) of the primary anti-CaSR antibody (5C10, ADD). The obtained data showed the absence of CaSR protein in hSC lines even at the higher concentration of the anti-CaSR antibody; indeed, with both antibody dilutions used, 1:500 and 1:100, the rate of stained cells is close to zero (0.27% and 0.41%, respectively), confirming the results obtained with immunofluorescence.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22147282
References
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2011, 13, 127–133.
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195.
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17.
- Berger, M.J.; Doherty, T.J. Sarcopenia: Prevalence, mechanisms, and functional consequences. Interdiscip. Top. Gerontol. 2010, 37, 94–114.
- Chang, N.C.; Chevalier, F.P.; Rudnicki, M.A. Satellite Cells in Muscular Dystrophy—Lost in Polarity. Trends Mol. Med. 2016, 22, 479–496.
- Dumont, N.A.; Rudnicki, M.A. Targeting muscle stem cell intrinsic defects to treat Duchenne muscular dystrophy. NPJ Regen. Med. 2016, 1, 16006.
- Morgan, J.; Partridge, T. Skeletal muscle in health and disease. Dis. Model. Mech. 2020, 13, dmm042192.
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 2014, 6, 246.
- Fu, X.; Wang, H.; Hu, P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. CMLS 2015, 72, 1663–1677.
- Jaroch, K.; Jaroch, A.; Bojko, B. Cell cultures in drug discovery and development: The need of reliable in vitro-in vivo extrapolation for pharmacodynamics and pharmacokinetics assessment. J. Pharm. Biomed. Anal. 2018, 147, 297–312.
- Gheller, B.J.; Blum, J.; Soueid-Baumgarten, S.; Bender, E.; Cosgrove, B.D.; Thalacker-Mercer, A. Isolation, Culture, Characterization, and Differentiation of Human Muscle Progenitor Cells from the Skeletal Muscle Biopsy Procedure. J. Vis. Exp. JoVE 2019, 150.
- Riddle, E.S.; Bender, E.L.; Thalacker-Mercer, A.E. Expansion capacity of human muscle progenitor cells differs by age, sex, and metabolic fuel preference. Am. J. Physiol. Cell Physiol. 2018, 315, C643–C652.
- Nehlin, J.O.; Just, M.; Rustan, A.C.; Gaster, M. Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism. Biogerontology 2011, 12, 349–365.
- Romagnoli, C.; Zonefrati, R.; Sharma, P.; Innocenti, M.; Cianferotti, L.; Brandi, M.L. Characterization of Skeletal Muscle Endocrine Control in an In Vitro Model of Myogenesis. Calcif. Tissue Int. 2020, 107, 18–30.
- Bareja, A.; Holt, J.A.; Luo, G.; Chang, C.; Lin, J.; Hinken, A.C.; Freudenberg, J.M.; Kraus, W.E.; Evans, W.J.; Billin, A.N. Human and mouse skeletal muscle stem cells: Convergent and divergent mechanisms of myogenesis. PLoS ONE 2014, 9, e90398.
- Kallay, E. Editorial: Physiology and Pathophysiology of the Extracellular Calcium-Sensing Receptor. Front. Physiol. 2018, 9, 413.
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51.
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947.
- Tu, M.K.; Levin, J.B.; Hamilton, A.M.; Borodinsky, L.N. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 2016, 59, 91–97.
- Avila, G. Disturbed Ca2+ Homeostasis in Muscle-Wasting Disorders. Adv. Exp. Med. Biol. 2018, 1088, 307–326.
- Cho, C.-H.; Woo, J.S.; Perez, C.F.; Lee, E.H. A focus on extracellular Ca2+ entry into skeletal muscle. Exp. Mol. Med. 2017, 49, e378.
- Brennan, S.C.; Thiem, U.; Roth, S.; Aggarwal, A.; Fetahu, I.S.; Tennakoon, S.; Gomes, A.R.; Brandi, M.L.; Bruggeman, F.; Mentaverri, R.; et al. Calcium sensing receptor signalling in physiology and cancer. Biochim. Biophys. Acta 2013, 1833, 1732–1744.
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842.
- Nemeth, E.F.; Shoback, D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 373–384.
- Sun, J.; He, W.; Bai, S.; Peng, X.; Zhang, N.; Li, H.; Zhang, W.; Wang, L.; Shao, X.; He, Y.; et al. The expression of calcium-sensing receptor in mouse embryonic stem cells (mESCs) and its influence on differentiation of mESC into cardiomyocytes. Differ. Res. Biol. Divers. 2013, 85, 32–40.
- Bikle, D.D.; Jiang, Y.; Nguyen, T.; Oda, Y.; Tu, C.-L. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front. Physiol. 2016, 7, 296.
- Mateo-Lozano, S.; García, M.; Rodríguez-Hernández, C.J.; de Torres, C. Regulation of Differentiation by Calcium-Sensing Receptor in Normal and Tumoral Developing Nervous System. Front. Physiol. 2016, 7, 169.
- Tharmalingam, S.; Hampson, D.R. The Calcium-Sensing Receptor and Integrins in Cellular Differentiation and Migration. Front. Physiol. 2016, 7, 190.
- Roesler, A.M.; Wicher, S.A.; Ravix, J.; Britt, R.D.; Manlove, L.; Teske, J.J.; Cummings, K.; Thompson, M.A.; Farver, C.; MacFarlane, P.; et al. Calcium sensing receptor in developing human airway smooth muscle. J. Cell. Physiol. 2019, 234, 14187–14197.
- Sambasivan, R.; Yao, R.; Kissenpfennig, A.; Van Wittenberghe, L.; Paldi, A.; Gayraud-Morel, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Dev. Camb. Engl. 2011, 138, 3647–3656.
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531.
- Atwood, B.K.; Lopez, J.; Wager-Miller, J.; Mackie, K.; Straiker, A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genom. 2011, 12, 14.
- Ziegelstein, R.C.; Xiong, Y.; He, C.; Hu, Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006, 342, 153–163.
- Tu, C.L.; Chang, W.; Bikle, D.D. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J. Biol. Chem. 2001, 276, 41079–41085.
This entry is offline, you can click here to edit this entry!
