Clostridioides difficile infection (CDI) has recently become a major healthcare-associated infection worldwide with great impact on healthcare systems as it evolves to a public health problem. The disease may develop due to multiple factors, including but not limited to different drugs usage, especially antibiotics and proton-pump inhibitors (PPIs), which interfere with intestinal flora promoting colonization and altering the immune status in particularly prone patients with inadequate nutritional status. Ischemic colitis (IC) results from diminished blood flow to the bowel wall and is the most frequently encountered type of intestinal ischemia. The ischemic injury can result in variable degree of colonic wall damage, ranging from superficial injury to full-thickness necrosis and perforation. IC mostly affects old female patients, and the clinical picture involves abdominal pain, diarrhea and hematochezia.
1. Case Report
A 30-year-old male patient without any history of diseases and without any prior treatments, was admitted in the Gastroenterology Department of the “Elias” Emergency University Hospital in Bucharest for hematochezia—three stools in the last 24 h, and colicky abdominal pain in the left lower quadrant. He denied experiencing unexplained weight loss or having similar symptoms before the present episode. His family history was negative for inflammatory bowel diseases and colorectal cancer. He denied the use of medication, drugs, alcohol consumption or smoking prior to hospital admission but his lifestyle anamnesis revealed an insalubrious workplace in a construction site with severely altered dietary habits regarding regular meals and adequate hydration. He was overweight, with a Body mass index (BMI) of 29 kg/m2.
Clinical examination upon admission revealed normal hemodynamic and respiratory parameters, normal temperature, with pain located in the lower left quadrant upon palpation but without any peritoneal signs.
Laboratory data showed leukocytosis with neutrophilia (16,000/μL, reference range 4600–10,200/μL), elevated C reactive protein at 4-times the upper limit of normal (22 mg/dL, reference range < 5 mg/dL), and low serum iron but without anemia. The stools were negative for parasites. Although the stool culture was negative, enzyme immunoassays for toxins A, B, and glutamate dehydrogenase (GDH) for the detection of CDI were all positive. Serum sodium level was 146 mmol/L (reference range 136–145 mmol/L) while serum osmolality 299 mOsm/kg. (280–300 mOsm/kg). The patient was started on oral Vancomycin 125 mg every 6 h associated with rehydration with intravenously administered crystalloids.
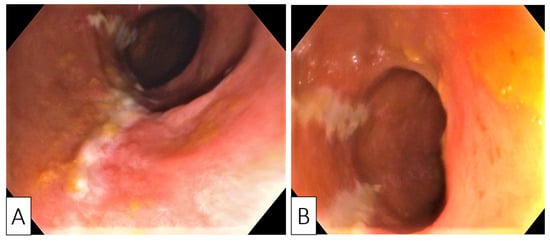
Despite no family history, sigmoidoscopy was performed to screen for inflammatory bowel disease or colorectal cancer, as there were no major risk factors for the development of CDI (e.g., use of antibiotics or prior hospitalization). Endoscopy revealed erythematous mucosa with longitudinal and parallel superficial ulcerations, in addition to luminal narrowing spanning from the junction of the sigmoid and descending colon. This narrowing precluded the advancement of the endoscope further—the appearance was highly suggestive for ischemic colitis (Figure 1A,B).
Figure 1. Endoscopic images showing longitudinal (A) and parallel (B) superficial ulcerations with surrounding mucosal edema and disappearance of colonic wall visible vascularization.
The procedure was prematurely abandoned due to the risk of perforation in this setting and biopsies were taken from the areas of ulceration.
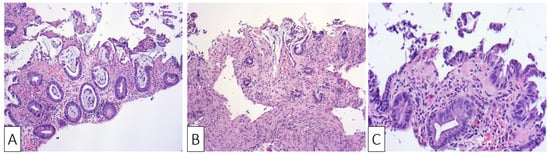
The histological examination confirmed the diagnosis of ischemic colitis describing colonic mucosa with surface epithelial degeneration, areas of mucosal necrosis and loss of superficial portions of glands (“withering crypts”). Lamina propria showed hyalinization, capillary congestion, focal hemorrhage, and reduced acute inflammation. The crypts were mucin depleted and the lining epithelium presented nuclear reactive changes consisting of nuclear hyperchromasia and enlargement, along with an increased number of mitotic figures (Figure 2A–C).
Figure 2. H&E ×10 Sigmoid mucosa at initial admission of the patient showing: (A) sloughing, epithelial regenerative changes, and focal hemorrhage with mild inflammatory changes; (B) sloughing, epithelial regenerative changes, and stromal hyalinization; (C) surface erosions and lamina propria hyalinization.
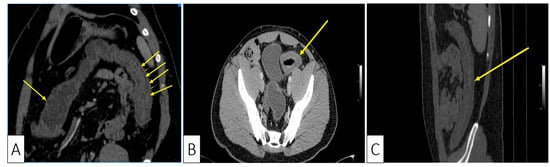
Consequently, we continued to investigate the patient with a computed tomography (CT) which showed a symmetrically thickened colonic wall corresponding to the descending and sigmoid segments, without obvious stenosis and without dilation of the proximal segments. The colonic vascularization on CT scan was negative for arterial or venous thromboses and the presence of the Comb sign was supportive of a local inflammatory process (Figure 3A–C).
Figure 3. Abdominal CT showing: (A) Coronal plane, oblique reconstruction image showing the difference between the normal wall (single arrow at the transverse colon) and the thickened pathological wall of the left colon (multiple arrows); (B) Axial plane, venous phase, showing stratified wall thickening of the proximal sigmoid; (C) Sagittal plane, showing the left colonic involvement with symmetrical wall thickening.
No supplementary treatments were added because the patient’s evolution was rapidly trending towards normalization of stools consistency, with disappearance of abdominal pain and rectal bleeding on the second day of treatment. The patient was discharged after 14 days of treatment with recommendations to perform a review colonoscopy in eight weeks. Additionally, he was advised to perform thrombophilia screening in an outpatient clinic.
He received no further medications after discharge.
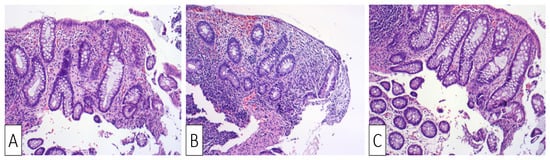
Upon readmission, the patient was clinically normal, his laboratory tests were normal, his weight continued to be stable, and his thrombophilia screening tests were negative. The colonoscopy showed a normal mucosa, with resolution of the prior stenosis, without any lesions throughout the entire colon and the last 15 cm of the terminal ileum. Biopsies were obtained from apparently normal mucosa at the site of prior ulceration, showing areas of lamina propria densification and residual predominantly lymphoplasmacytic inflammation, along with focal architectural changes (Figure 4A–C).
Figure 4. H&E ×10 Sigmoid mucosa at follow-up following treatment: (A) Sigmoid mucosa showing mild distortion of crypts and lamina propria densification.; (B) sigmoid mucosa showing distortion of crypts and lamina propria densification; (C) sigmoid mucosa showing lamina propria densification (top right) compared to normal stroma (bottom left).
2. Discussions
The evolution process of societies caused by the industrial revolution has contributed to enormous advance in medicine with concurrent increase in life expectancy, but it has also altered disease evolution patterns in these populations. The modern lifestyle leads to the eradication of nonpathogenic bacteria through limited beneficial bacteria intake, along with ageing process and dietary changes as contributing factors for the development of altered gut microbiota [
18].
The individual flora diversity is influenced by multiple circumstances such as acute diarrhea, PPIs, NSAIDs and antibiotic treatments, or to a lesser extent, dietary excess. These factors can alter gut microbiota, either alone or in combination, generating changes in the intestinal microenvironment that make the host susceptible to the action of pathogenic agents.
The most studied aforementioned factor is represented using antibiotics which have been shown to influence gut microbiota through either interference with mutualistic interaction between different community members, or direct toxic effect on others [
19]. Thus, the loss of the intestinal environmental microbial equilibrium created the premises for the development of bacterial species such as
Clostridium difficile.Until now, two cases have been described in the literature with ischemic colitis and CDI colitis in the same patient.
Most frequently, ischemic colitis is caused by non-occlusive ischemic injury to the bowel wall through sudden decrease in blood supply in the small vessels of the colon, usually secondary to a low circulating volume state [
20,
21]. There are several well-established risk factors that predispose towards the development of ischemic colitis, such as atherosclerosis, aortic surgery, oral contraceptives, hereditary coagulopathies, cocaine abuse, infectious colitis caused by Cytomegalovirus (CMV) and
Escherichia coli [
22]. In our patient, none of the above-mentioned predisposing factors were encountered.
Regarding the anatomical distribution of ischemic colitis, the disease has been classified as left- and right-sided. The former is usually associated with diminished blood flow, coagulopathies, cardiac and aorta surgery, while the latter is generally caused by superior mesenteric artery obstruction [
23]. Our patient presented with a left-sided ischemic colitis in the absence of any history of surgery and testing negative for thrombophilia, with the only remaining causative factor related to dehydration.
The set of predisposing factors for onset of ischemic colitis early in life has been studied and found to be distinct from disease affecting older patients—smoking was found to be the most important risk factor.
Dehydration is defined by an increase in serum osmolality above normal values (275–295 mOSm/kg) and is classified into impending and current based on the severity of serum osmolality increase (impending dehydration 295–300 mOsm/kg, current dehydration > 300 mOsm/kg) [
24,
25]. It results mainly through insufficient fluid intake and excessive loss in vomiting and diarrhea. In our case, the impeding dehydration at admission might have been caused by insufficient intake developing intro current dehydration through diarrhea.
There has been identified an entity mimicking ischemic colitis in long-distance runners—called “runner’s colitis”, which is also presumed to be an ischemic colitis caused by dehydration [
15,
26]. In our case, the patient denied smoking and did not declare any recent strenuous physical effort which may have contributed to dehydration.
In stark contrast, CDI has been a topic of rigorous research and risk factors, such as recent hospitalization and prior antibiotic use, are well-described risk factors for the development of colitis. However, none of these factors were present in our patient.
The deleterious progression to severe colitis in CDI is well-described in the literature and involves a mixed infectious and ischemic insult to the colonic mucosa. The mechanism implicates a stepwise exacerbation secondary to dehydration and hypotension precipitated by severe diarrhea induced by CDI, which leads to either global or localized bowel ischemia. This contributes to a more pronounced systemic inflammatory response and subsequently, fulminant colitis [
27,
28,
29].
This entry is adapted from the peer-reviewed paper 10.3390/medicina57070705