Critically ill patients have an alteration in the microbiome in which it becomes a disease-promoting pathobiome. It is characterized by lower bacterial diversity, loss of commensal phyla, like Firmicutes and Bacteroidetes, and a domination of pathogens belonging to the Proteobacteria phylum. Critically ill patients also have a hyperpermeable gut barrier and dysregulation of the inflammatory response that favor the development of the pathobiome, translocation of pathogens, and facilitate the emergence of sepsis.
1. Introduction
The microbiome has been intensely studied and the understanding of its metabolic and immunological functions has had remarkable advances. The disruption of the microbiome homeostasis, known as “dysbiosis” or “pathobiome”, can be as important as the host genetics for the development of various conditions, such as inflammatory bowel disease, obesity, diabetes, or cardiovascular disease. In critically ill patients, who are affected by a life-threatening multisystem process that can result in significant morbidity or mortality [
1], many factors can contribute to the development of a pathobiome, including intrinsic factors, like genetics or age, and those that can be manipulated by either the human host or medical interventions, such as diet, geographic location, or drug therapy [
2,
3,
4]. Lately, special attention has been paid to the relationship between nutrition and the microbiome, but more data is needed to understand which nutrients participate in the maintenance of the microbiome homeostasis in health and disease, and which interventions could help to recover this homeostasis during and after critical illness, like nutritional supports or the use of probiotics, prebiotics, and fecal transplantation. The aim of this review is to present the current knowledge about the role of the microbiome in critically ill patients and the modulatory role of nutrition, which can determine their evolution and the efficacy of the current therapeutic strategies.
2. The Gut Microbiome
The human gut contains more than 1000 different microbial species that collectively encode over 100 times more genes than the human genome [
5]. In healthy humans, the intestinal microbiome is composed of members of the three domains of life—bacteria, archaea, and eukaryotes, although the bacterial community is the most abundant and heterogeneous. Nine different bacterial phyla have been reported, with Bacteroidetes and Firmicutes being the most dominant members [
6,
7,
8].
It is difficult to characterize all the populations since many of them cannot be grown in vitro. However, recent advances in the culture methods for "non-cultivable" human microbes have revealed a whole spectrum of new species and bacterial taxa [
9]. Moreover, techniques such as 16S rRNA and shotgun metagenomic sequencing have opened a new area of research, allowing for the identification of complex populations of bacteria, and their effects on health and disease [
10,
11].
3. The Gut Microbiome in Critically Ill Patients
3.1. Changes in the Gut Microbiota in Critically Ill Patients
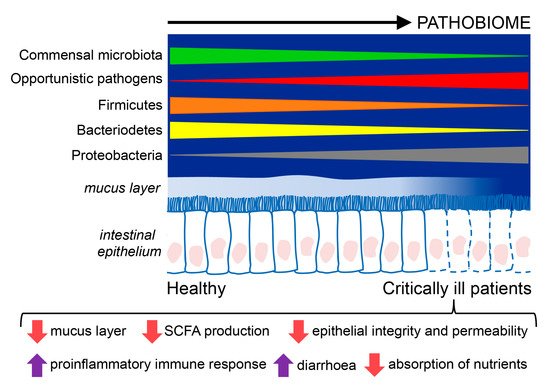
The intestine has long been hypothesized as "the engine" of critical illness, but its clinical importance needs to be better defined. The gut microbiome is severely altered in multiple disease states, including critical illness, where the health-inducing microbiome becomes a disease-promoting pathobiome that makes the patient more vulnerable to nosocomial infections, sepsis, and multiple organ failure [
20]. So far, only a few studies have analyzed the gut microbiome in critically ill patients, and they have confirmed a state of dysbiosis [
21,
22,
23]. Moreover, recent studies in intensive care unit (ICU) patients observed a gradual worsening of the dysbiosis during their stay in the ICU [
22,
24,
25,
26]. The most relevant changes in the microbiome can be seen in the largest study to date that examined the sequencing of the 16S rRNA gene from multiple body sites (skin, oral, and feces) from 115 ICU patients and compared it with 1242 healthy volunteers [
22]. At the intestinal level there was a low prevalence of the Firmicutes and Bacteroidetes phyla, and a greater richness of Proteobacteria in comparison to healthy individuals. At the genus level, there was a lower prevalence of key commensal genera (such as
Faecalibacterium—an anti-inflammatory organism,
Blautia, and
Ruminococcus), and in some cases, an overgrowth (over 50% relative abundance) of genera with pathogenic properties, such as
Escherichia/
Shigella,
Salmonella,
Enterococcus,
Clostridium difficile, or
Staphylococcus [
21,
22,
23,
24,
27]. It has been proposed that changes in the
Firmicutes/Bacteroidetes ratio can predict patient outcome [
24], although further work is required to validate these findings.
Overall, critically ill patients admitted to the ICU present a gut microbiome characterized by lower bacterial diversity and large inter-individual variation. A study of 14 ICU patients also reported the emergence of ultra-low-diversity communities in 35% of patients who only presented one to four bacterial taxa [
27]. In previous studies, low microbial diversity has been associated with an increased risk of mortality [
28,
29], and the domination of certain pathogens have been identified as an independent risk factor for adverse outcomes [
30,
31,
32].
Considering all the studies on critically ill patients, Proteobacteria is the dominant phyla, and Firmicutes is reduced, whereas Enterococcus, Staphylococcus, and Enterobacter are increased in septic patients. In these patients with sepsis, the focus often lies on the identification of a single pathogen as the causative agent. However, there is an increasing belief that most infections have “polymicrobial” phenotypes that depend on the microbiome status of the patient. Thus, the initial state of the microbiome can determine both the susceptibility to infection [
33] and its severity [
34]. The composition and functions of the intestinal microbiome of critically ill patients and healthy humans are summarized in
Figure 1.
Figure 1. Composition and functions of the intestinal microbiome in critically ill patients compared to healthy individuals. Critically ill patients exhibit an intestinal disease-promoting microbiome or pathobiome. This pathobiome is characterized by a lower prevalence of the Firmicutes and Bacterioidetes phyla, and a higher prevalence of the Proteobacteria phyla, in contrast to healthy individuals. Furthermore, the intestinal epithelium is altered in critically ill patients, showing reduced reperfusion, that could lessen the hydrophobicity of the mucus layer and favor the translocation of pathogens through gaps between the epithelial cells, and epithelial apoptosis, resulting in poor absorption of nutrients, diarrhea, loss of fecal energy, and lower production of short chain fatty acids (SCFA).
Furthermore, migration of microorganisms between the intestinal and pulmonary microbiome has been reported in critically ill patients. A recently published study highlights the impact of the gut microbiome on the pulmonary microbiome. It was observed that the pulmonary microbiome in both murine sepsis and human acute respiratory distress syndrome (ARDS) was enriched with bacteria associated with the intestine. An operative taxonomic unit of Bacteroides was detected in the bronchoalveolar fluid (BAL) samples from 41% of patients with ARDS compared to 3% in healthy patients. Moreover, the systemic and alveolar levels of tumor necrosis factor (TNF)-α in patients with ARDS were markedly increased by the presence of organisms derived from the intestine in the BAL. However, the precise route by which the intestinal microorganisms reached the lungs of the mice with sepsis has not been identified [
35].
3.2. Modulators of the Microbiome in Critical Illness
The alteration of the microbiome in critically ill patients is multicausal. Critical disease leads to profound modifications in the gut microbiome, caused by general alterations in the host environment [
36,
37] including enhanced virulence of the bacteria due to the expression of ancestral or newly acquired genes [
38].
In addition, many treatments administered to patients in the ICU, like antibiotics, proton pump inhibitors, vasopressors, and opioids, produce harmful effects outside their target organ, which directly affect the microbiome. The most significant alterations are probably related to antibiotic treatments since they indiscriminately ablate the commensal microbiome, favoring the intrusion of secondary pathogenic microorganisms and the enrichment of antibiotic resistance genes [
39]. Thus, antibiotic therapies may aggravate the alteration of the microbiome caused by the different pathologies. In fact, the use of antibiotics in ICUs is very frequent, with 71% of the patients receiving antibiotic treatment, according to data from the Centers for Disease Control and Prevention in the United Sates [
40]; although it is estimated that 35% of the antibiotic regimens are unnecessary, according to the latest recommendations. As a result, the increase in mortality and morbidity associated with these alterations leads to an additional increase in the cost and care related to the ICU.
Besides, the inappropriate use of antibiotics is considered to be responsible for the increasing emergence of multidrug-resistant bacteria (MDR), and it is important to consider that nosocomial infections represent an additional complication in critically ill patients. The incidence of MDR infections is escalating rapidly all over the world [
41]. In fact, a recent publication calculated that infections with antibiotic-resistant
Clostridium difficile occur in more than 450,000 patients per year, in the United States [
41]. In addition, MDR infections are increasingly lethal for hospitalized patients. It is calculated that
C. difficile contributes to more than 30,000 deaths per year in the United States [
41,
42]. Consequently, the implementation of new antibiotic drugs has not significantly improved the survival to infectious diseases in developed countries, but has instead contributed to the emergence of increasingly aggressive MDR organisms.
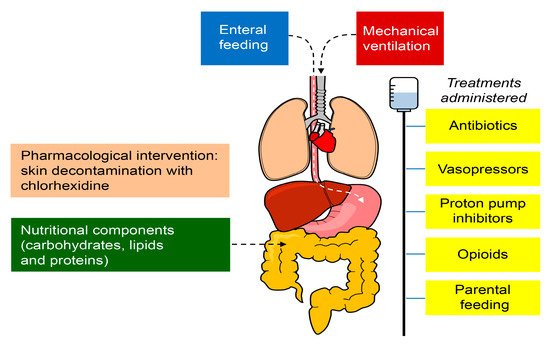
Moreover, nutrition is another key factor for the gut microbiome homeostasis since it primarily depends on the availability of enteral nutrients for survival. Thus, the nutritional components (carbohydrates, lipids, and proteins) and the route of administration (enteral/parenteral) might also alter the health of the microbiome [
43,
44,
45]. In addition, pharmacological interventions can modify the specific conditions of the body site (for example, skin decontamination with chlorhexidine) and invasive procedures may alter the natural barrier mechanisms (e.g., endotracheal intubation, intravascular catheters) that could facilitate the access and proliferation of microbes [
46]. The factors that may alter the microbiome are shown in
Figure 2. Therefore, the impact of ICU care on the microbiome should be further explored.
Figure 2. Factors that may alter the microbiome in critically ill patients in the ICU. The treatments administered to patients in the ICU, including antibiotics, proton pump inhibitors, vasopressors, and opioids, produce harmful effects outside their target organ, which directly affect the microbiome. The nutritional components (carbohydrates, lipids, and proteins) and the route of administration (enteral/parenteral) might also alter the health of the microbiome. The pharmacological interventions can modify the specific conditions of the body site (for example, skin decontamination with chlorhexidine) and invasive procedures may impair the natural barrier mechanisms (e.g., endotracheal intubation and intravascular catheters), facilitating the access and proliferation of microbes.
3.3. Epithelial Alterations and Intestinal Hyperpermeability in Critically Ill Patients
Critical illness induces hyper-permeability of the gut barrier that begins as early as one hour after the onset of sepsis or trauma and lasts for at least 48 h [
47,
48]. Mucus also plays a crucial role in the defence of the host by preventing bacteria and digestive enzymes from contacting the intestinal epithelium as a result of mucus hydrophobia that reduces the absorption of toxic molecules. In critical disease, the mucus layer is affected, which induces the dysfunction of epithelial cells. Actually, in these patients, it is very common to find a reduced intestinal reperfusion that can lessen the hydrophobicity of the mucus layer and alter the intestinal permeability [
49].
One of the mechanisms responsible for the epithelial defects in critically ill patients could be the impairment of short chain fatty acids (SCFA) production. One important metabolic function of the gut microbiome is the fermentation of dietary fiber and production of SCFA, including butyrate, which serves as the primary energy source for the colonic epithelium and preserves its integrity [
50]. During sepsis, a rapid and persistent fall in the concentration of SCFA takes place [
51] and as a consequence, the mucosal epithelial barrier is impaired due to epithelial apoptosis resulting in poor absorption of nutrients, diarrhea, loss of fecal energy, and pathogen translocation [
22,
23]. However, it has been described in a graft versus host disease mouse model that when bacterial strains, which are capable of producing large amounts of SCFA, are ingested, the severity of the disease decreases. This is explained by the high intestinal concentrations of butyrate that improve the epithelial barrier by enhancing intercellular junctions and decreasing cell apoptosis [
52].
3.4. Relevance of the Gut Microbiome in Critical Illness
Considering the microbiome as an internalized organ with important physiological functions, it is evident that its alteration might be as harmful as other “organ failures” in ICU patients. The possible damage could be caused by both the loss of “organ” function and also the aberrant physiology replacing its function. In this context, the “lost organ” is the commensal microbial community that helps to metabolize drugs, nutrients, and hormones, modulate immune responses, and maintain the mucosal barrier homeostasis. By losing commensal microbes, the host also loses protection against invading pathogens by different mechanisms. The gut microbiome is the main activator of the host immunity against infections, which involves innate (stimulation of granulopoiesis, production of antimicrobial peptides (bacteriocins), and nutrient source competition) [
39,
50] and adaptive (regulation and differentiation of Th17 cells) mechanisms [
53]. The “aberrant physiology” is represented by emerging pathogens that dominate microbial communities and cause dysregulated inflammatory responses, excessive inflammation leading to multiple organ dysfunction, and eventually, immune depletion due to the loss of specific microbial signals necessary for the maintenance of normal T cell function in the gut, that could facilitate the emergence of super-infections [
54] and ultimately sepsis [
55].
Hence, recent preclinical data derived from animal models suggest that the intestinal microbiome plays a protective role in the host defence against sepsis [
34,
58,
59]. In murine models of Gram-positive and Gram-negative pneumosepsis, it has been shown that antibiotics can induce the disruption of the gut microbiome, which increases inflammation and bacterial spread [
34,
59]. Similarly, data from ICU patients indicate that the loss of microbiome diversity implies an increased length of stay in the ICU, which further highlights the potential clinical relevance of the intestinal microbiome for critically ill patients [
22].
4. Nutrition of the Critically Ill Patient
Medical nutritional therapy in critically ill patients is a challenge due to the great heterogeneity among patients and the variable duration of the acute phase of disease, firstly characterized by hemodynamic instability and a severe increase in catabolism that later progresses to a period of muscle wasting and stabilization of the metabolic alterations [
60]. The management of critically ill patients and the outcome of disease could be notably improved by monitoring their metabolic profile (protein-energy malnutrition, lipidome, and the levels of glucose, insulin, vitamin D
3, and other micronutrients) [
61,
62] and implementing an individualized nutritional treatment. There are many guidelines for the care of critically ill patients, but the supporting studies lack external validity due to the heterogeneity of patients, resulting in no general agreement in the nutritional recommendations. Thus, further investigation is needed to achieve a consensus to guide clinicians [
63,
64].
Many studies have shown that the lack of enteral nutrition, which is very frequent in the ICU, may alter the intestinal microbiome composition and weaken the epithelial barrier function, predisposing it to bacterial translocation, which is also associated with septic complications [
65,
66].
For instance, the production of butyrate, the main energy source for intestinal epithelial cells, would be compromised since it is produced by the microbiome fermentation of dietary fibers in the large intestine. Recent experimental studies have demonstrated how this vicious cycle begins and that the gastrointestinal tract responds with an elaborate system of regulatory mechanisms that can be altered when enteral nutrients are absent [
67].
“The ESPEN guidelines” have been published in order to offer the best medical nutritional therapy to ICU patients [
60]. They recommend medical nutritional therapy for all patients admitted to the ICU, and particularly to those staying for more than 48 h, including administration of oral nutritional supplements, enteral nutrition, and parenteral nutrition. Moreover, they encourage the use oral nutrition, but when it is not possible, enteral nutrition should be initiated within 48 h.
Enteral nutrition has greater benefits in the critically ill patient, making it the preferred modality in patients with a functioning gastrointestinal tract. The main benefit lies in the activation of luminal detection mechanisms, which stimulate and modulate the cellular activities of the mucosa. However, many critically ill patients cannot receive enteral nutrition due to intolerance problems or clinical conditions where enteral nutrition is contraindicated according to the European Society of Intensive Medicine (ESCIM) [
68] (
Table 1).
Table 1. Enteral Nutrition in special conditions.
|
Enteral Nutrition in Special Conditions
|
|
Early EN should be implemented
|
Low dose EN should be administered
|
EN should be delayed
|
|
Patients receiving ECMO
|
Patients with therapeutic hypothermia
|
Patients with uncontrolled shock (when hemodynamic and tissue perfusion goals are not reached)
|
|
Patients with traumatic brain injury
|
Patients with intra-abdominal hypertension without abdominal compartment syndrome
|
Patients in uncontrolled life-threatening hypoxemia, hypercapnia or acidosis
|
|
Patients with stroke (ischemic or hemorrhagic)
|
Patients with acute liver failure
|
Patients suffering from active upper gastrointestinal bleeding
|
|
Patients with spinal cord injury
|
|
Patients with overt bowel ischemia
|
|
Patients with severe acute pancreatitis
|
|
Patients with high-output intestinal fistula
|
|
Patients after gastrointestinal surgery
|
|
Patients with abdominal compartment syndrome
|
|
Patients after abdominal aortic surgery
|
|
Patients with gastric aspirate volume above 500 mL/6 h.
|
|
Patients with abdominal trauma while the continuity of the gastrointestinal tract is restored
|
|
|
|
Patients delivery neuromuscular blocking agents
|
|
|
|
Patients managed in prone position
|
|
|
|
Patients with an open abdomen
|
|
|
Note: Recommendations published by the European Society of Intensive Medicine (ESCIM) for the initiation of early enteral nutrition (within 48 h of Intensive Care Unit admission) and recommendations favoring delaying it [63]. EN (enteral nutrition), ECMO (ExtraCorporeal Membrane Oxygenation).
This entry is adapted from the peer-reviewed paper 10.3390/nu11123002