The insulin signaling pathway begins with the binding of the peptide hormone insulin to its corresponding receptor, the insulin receptor. The insulin receptor is a receptor tyrosine kinase (RTK) that conformationally consists of two alpha and two beta subunit tetramers. The insulin receptor exhibiting kinase activity is responsible for its autophosphorylation at the tyrosine residue site upon insulin binding.
- insulin resistance (IR)
- type 2 diabetes mellitus (T2DM)
- branched-chain amino acid (BCAAs)
- metabolic dysfunction
- gut microbiome
- inflammation
- mammalian target of rapamycin (mTOR)
- G-protein coupled receptor (GPCRs)
1. Introduction
Ample amounts of basic macromolecules such as carbohydrate, protein, and fat are crucial for optimum bodily function. Insulin resistance was proven in numerous studies to be the onset of many metabolic disorders in humans, especially diabetes mellitus [1][2]. An elevated concentration of certain plasma amino acids was intricately linked as one of many diverse factorial precursors in the development of diabetes. Branched-chain amino acids (BCAAs) have been found to be escalated in people with insulin resistance, and therefore may serve as possible biomarkers in the early detection upon onset of the disease [3][4][5].
The status of diabetes has changed from being classified as a subdued disorder of elderly people to being a major cause of morbidity and mortality affecting the youth and middle-aged people [6][7]. According to the Diabetes Atlas 9th edition 2019, published by the International Diabetes Federation, there are around 462 million people living with diabetes globally, and the number is expected to rise unless urgent preventive measures are taken. T2DM is characterized by the amount of blood sugar/glucose (mg) per deciliter (dL) of blood. Normal blood sugar levels measured with a fasting blood glucose test are ≤99 mg/dL, while any reading greater than 125 mg/dL is associated with a higher risk of developing insulin resistance, or worse, diabetes [8].
Insulin resistance indicates the inability or inefficiency of the body to respond well to hormone insulin [9]. The pancreatic islet of Langerhans consists of three types of cells, i.e., alpha cells, beta cells, and delta cells. Peptide hormone insulin is secreted by β-cells of the islet, which function as messengers that instruct designated peripheral insulin-sensitive cells in the body to take up glucose. Any defect that interferes with this process would potentially promote malfunction of the pancreas, because constant ectopic production of insulin is needed to compensate lower blood sugar levels [10].
Branched-chained amino acids (BCAAs) and aromatic amino acids (AAAs) were shown to be associated with insulin resistance and may serve as possible markers prior to the onset of diabetes. Upon comparison with the metabolic marker homeostatic model assessment of insulin resistance (HOMA-IR) for diabetes, recent studies provide evidence that BCAAs and AAAs are closely correlated with the progression of T2DM [2][3][4][5][11][12][13]. BCAAs are essential amino acids that include leucine, isoleucine, and valine and account for 40% of total amino acids needed by mammals [14][15]. The tampering of BCAAs in the impairment of insulin action was broadly reviewed as a consequence from the downregulation of BCAA metabolism in muscle controlled by the mTOR pathway, which is known to be regulated by insulin and leucine.
The direct and accurate elucidation correlating to the actual involvement of AAAs with the impairment of insulin action still demands more research. A study in 2016 examined the global transcript profile of adipose tissue and skeletal muscle; the peripheral cells and the microarray analysis results revealed the upregulation and downregulation of certain genes. Upregulated genes are involved in the adaptive immune response, which scans for the inflammation and infiltration of inflammatory cells, both of which induce insulin resistance over time. The downregulated genes are those responsible for the metabolism of AAAs, which evidently explains the etiology behind the elevated plasma concentration of AAAs in insulin
Faulty insulin receptor activity often breeds many metabolic complications in consideration that insulin influences the majority of cellular metabolism processes. Moreover, metabolic disorders such as insulin resistance are eminently known to be provoked through impaired action of this receptor [9][16]. At present, the G-protein-coupled receptor (GPCR) highlights its ability to induce the insulin signaling pathway (Figure 1) independent of insulin perception, which is mediated through cross-talk with the insulin receptor [17][18][19][20]. A detailed review on the association of amino acids with the prevalence of insulin resistance is presented in this review.
2. Insulin Resistance and Type 2 Diabetes Mellitus
Notably, there are various factors recognized to trigger insulin resistance (Figure 2): for instance, obesity [21][22][23][24], critical hyperglycemia [7][25], low-grade inflammation [23][24], the overproduction of reactive oxygen species (ROS) [25][26], mitochondrial dysfunction [27], impaired insulin signaling pathway [28], short-chain fatty acids (SCFAs) [29], and amino acids [2][4][30][31]. One study by Jenganathan et al. showed that amino acids are implicated with IR, although this is rather temporal and reversible. The incubation of myotubes with amino acids and insulin significantly increases the level of phosphorylation and activation of the mTOR pathway; however, this is reversible as well [31].
Nevertheless, recent lifestyle advancements have altered this phenomenon; nowadays, the onset of T2DM can be observed in children and middle-aged people [7]. Early detection and preventative measures need to be addressed immediately to avert this exacerbation of T2DM, because any delayed attention could breed ugly complications. [32], the fasting plasma glucose (FPG) test [33], fasting plasma insulin (FPI) test [10], oral glucose tolerance test (OGTT) Unchecked T2DM coincides with a myriad of complications and paradoxical effects including retinopathy, neuropathy [6][12], neointimal hyperplasia, microvascular and macrovascular disease [6][34], permanent pancreas dysfunction [10], etc.
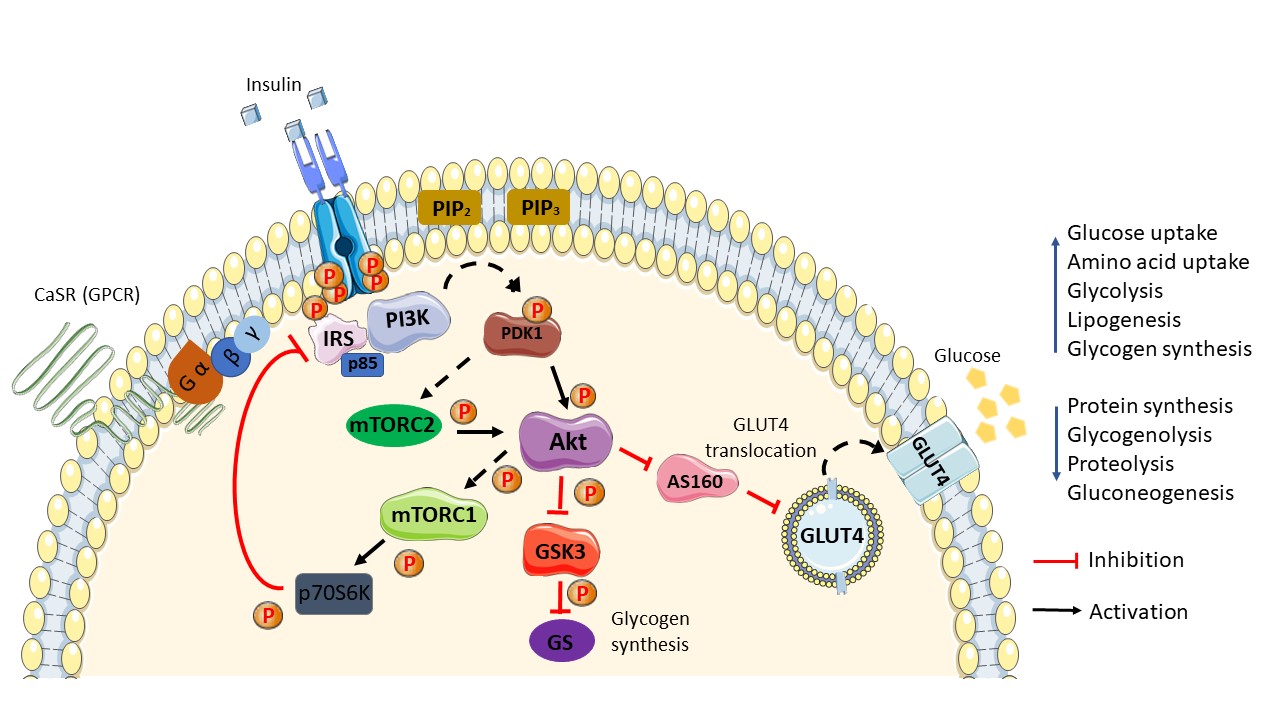
Figure 1. Schematic diagram of the general insulin signaling pathway in cells mediated through IR/RTK. The phosphorylation of RTK recruits various downstream regulatory proteins. The insulin signal is transduced among the target proteins/enzymes until GLUT4 vesicle fusion with the plasma membrane of the cell: CaSR, calcium-sensing receptor; IRS, insulin receptor substrate; p85, phospho-PI3 kinase p85; PI3K, phosphoinositide 3-kinases; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-bisphosphate; PDK1, phosphoinositide-dependent protein kinase-1; mTORC (1/2), mammalian/mechanistic target of rapamycin complex (1/2); Akt, protein kinase B; AS160, Akt substrate of 160 kDa; GLUT4, glucose transporter 4; GSK3, glycogen synthase kinase; GS, glycogen synthase; p70S6K, ribosomal protein S6 kinase beta-1 (S6K1); P, phosphate. Phosphorylation activation (black arrow). Phosphorylation inhibition (red arrow).
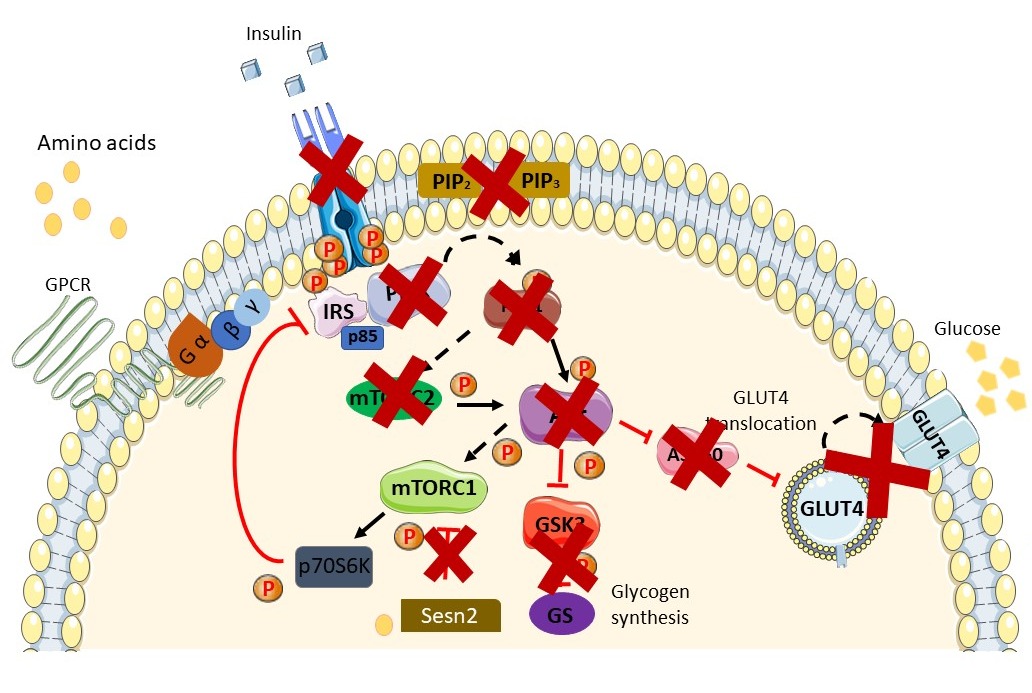
Figure 2. Schematic diagram of the insulin-resistant state. Available peptide hormone insulin failed to activate IR/RTK. Downstream signaling of the insulin signaling pathway is blunted in the insulin-resistant state and in the prevalence ofT2DM. Augmentation in AAs in the insulin-resistant state further exacerbates the condition via activation of the mTORC1 pathway through AA (leucine) binding with Sestrin2. Activation of the mTOR pathway induces phosphorylation inhibition on serine residues of IRS1/2 and causes insulin resistance. A more thorough explanation of the involvement of AAs is found in the review. Sesn2, Sestrin2.
3. G-Protein Coupled Receptor Signaling
G-protein coupled receptors (GPCRs) are the largest plasma membrane receptor targeted by a myriad of drugs approved by the FDA [35][36]. GPCRs are characterized through their infamous seven transmembrane domain, spanning the plasma membrane with its extracellular N terminal forming complementary pockets where ligands bind, with an intracellular C terminal domain that bridges the receptor to its G-protein domains, i.e., Gα, Gβ and Gγ. GPCRs recognize a broad variety of ligands including chemicals, hormones, and proteins, portraying its fair share as an important initial regulator to virtually all physiological activities in the body. Each group shares a similar signal activation mechanism that, upon binding of the ligand with the receptor and conformational alteration, the Gα subunit which was previously bound to GDP (inactive) now binds to GTP and dissociates itself from the complexes to start the signaling cascade.
4. Involvement of G-Protein Coupled Receptor
Reassuring an unflawed cell signaling pathway is a complex process that requires the engagement of various regulatory molecules (ligands, receptors, and responses). Cross-talk between receptors has been acknowledged for decades and is required to accomplish a good signaling transduction pathway. Nevertheless, a study in 2017 suggested that GPCR angiotensin receptor type 1 (AT1R) and β-adrenergic receptors (βARs) are associated with the insulin signaling pathway through the PI3K pathway and RAS/RAF/MEK/ERK1/2 signaling cascade, respectively [37]. The nutrient sensing receptor falls under class C GPCR, which is characterized by a large Venus flytrap (VFT) ligand-binding extracellular domain with obligate dimerization action (homo or heterodimer) and promiscuous ligand selection [38].
is a G-protein coupled receptor described by various publications to sense phenylalanine derivatives and tryptophan as its agonist and is abundantly expressed on β-cells of the pancreas. GPR142, activated by tryptophan, is a natural precursor to niacin and serotonin. The activation of this GPCR enhances glucose-stimulated insulin secretion. Recent publications have highlighted that the increased level of tryptophan in more serious IR subjects displayed positive outcomes after inactivation of the receptor knockdown in mice, and observations with its antagonist (CLP-394) have proven beneficial [39][40].
Class C GPCR, GPRC6A, responds to L-amino acids such as lysine and arginine, which bind to its large Venus flytrap N terminal extracellular receptor. GPRC6A is also seen to respond to decarboxylated osteocalcin (OCN), which improved insulin activity in wild-type mice while providing the opposite effect inGpcr6a−/−mice. This receptor is reproduced in numerous animal species [41].
After its discoveries, T1R2 and T1R3 have been intimately assigned to potentially regulate nutrient uptake and digestion. Consequent to dietary carbohydrate and protein consumption, a higher expression of T1R3, T1R2 and α-gusductin is noticed in conjunction with the available nutrients [42][43]. In pancreatic β-cells, AA binding to T1R1/T1R3 stimulates the MAPK ERK1/2 pathway, which is known to mediate insulin expression and exocytosis via the nutrient-dependent insulin gene transcription. Incretins function upon taste perception to better facilitate digestion through enhanced insulin secretion, the suppression of glucagon secretion, and overall improvements in satiety.
An ex vivo functional study of insulin receptors of rat taste cells in organoids disclosed that the genetic expression of proteins related to the taste cells was significantly reduced (gustducin, T1R3, SGLT, and GLUT) in an insulin-enriched environment. Reminiscent to the above notion, treatment with rapamycin did naturally reverse faulty gene expression, and normal amounts of taste cells were restored [44]. Parallel to justifying the above hypothesis, cells with defects in their taste perception were observed to upregulate the genetic expression of mandatory proteins (transport proteins) to normalize the situation [45]. Therefore, it is concluded that the availability of taste receptors is governed by nutrient availability.
Calcium-sensing receptor (CaSR) is a class C GPCR that supervises parathyroid hormone (PTH) secretion via extracellular calcium concentration and calcium absorption at the kidney [46]. Apart from calcium, CaSR also responds to amino acids, and previous studies have shown that CaSR is involved in glucose hemostasis [47]. As discussed earlier in the paper, activation of the mTOR pathway negatively blunted insulin signaling, whereas taste perception continuously induced insulin secretion through the AMPK pathway; Although evidence of the direct involvement of CaSR on amino acid-induced insulin resistance has not yet been reported, the plausibility is there, because the receptor is also activated by amino acid.
function to induce insulin secretion, suppress glucagon action, and improve satiety. AA drinks administered orally resulted in higher insulin secretion and action in comparison to AAs administered intravenously A whey protein diet improved satiation significantly due to its amino acid constituents, displaying improved insulin action and decreased hunger [48]. The above situation can be intimately linked to the nutrient-sensing ability of class C GPCRs, because the oral administration of BCAAs induces incretin secretion, which helps in overall satiation and increases insulin response, whereas intravenous BCAA does not [49].
This entry is adapted from the peer-reviewed paper 10.3390/nu13072229
References
- Pieter Giesbertz; Hannelore Daniel; Branched-chain amino acids as biomarkers in diabetes. Current Opinion in Clinical Nutrition & Metabolic Care 2016, 19, 48-54, 10.1097/mco.0000000000000235.
- Yanislava Karusheva; Theresa Koessler; Klaus Strassburger; Daniel Markgraf; Lucia Mastrototaro; Tomas Jelenik; Marie-Christine Simon; Dominik Pesta; Oana-Patricia Zaharia; Kálmán Bódis; et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. The American Journal of Clinical Nutrition 2019, 110, 1098-1107, 10.1093/ajcn/nqz191.
- Kenji Nagao; Minoru Yamakado; The role of amino acid profiles in diabetes risk assessment. Current Opinion in Clinical Nutrition & Metabolic Care 2016, 19, 328-335, 10.1097/mco.0000000000000305.
- Miwa Miwa Kawanaka; Ken Nishino; Takahito Oka; Noriyo Urata; Jun Nakamura; Mitsuhiko Suehiro; Hirofumi Kawamoto; Yasutaka Chiba; Gotaro Yamada; Tyrosine levels are associated with insulin resistance in patients with nonalcoholic fatty liver disease. Hepatic Medicine: Evidence and Research 2015, ume 7, 29-35, 10.2147/hmer.s79100.
- Tianlu Chen; Yan Ni; Xiaojing Ma; Yuqian Bao; Jiajian Liu; Fengjie Huang; Cheng Hu; Guoxiang Xie; Aihua Zhao; Weiping Jia; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Scientific Reports 2016, 6, 20594, 10.1038/srep20594.
- Nita Forouhi; Nicholas J. Wareham; Epidemiology of diabetes. Medicine 2014, 42, 698-702, 10.1016/j.mpmed.2014.09.007.
- Diabetes: Mechanism, Pathophysiology and Management-A Review . International Journal of Drug Development & Research. Retrieved 2021-7-10
- Luciana B. Nucci; Cristiana M. Toscano; Ana Lourdes M. Maia; Cláudio D. Fonseca; Maria Moema B. Britto; Bruce B. Duncan; Maria Inês Schmidt; A nationwide population screening program for diabetes in Brazil. Revista Panamericana de Salud Pública 2004, 16, 320-327, 10.1590/s1020-49892004001100005.
- Karyn J. Catalano; Betty A. Maddux; Jaroslaw Szary; Jack F. Youngren; Ira D. Goldfine; Fred Schaufele; Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain. PLOS ONE 2014, 9, e108693, 10.1371/journal.pone.0108693.
- Yin-Yi Ding; Xiang-Rong Cheng; Zhu-Qing Li; Sha-Ji Wu; Yuhui Yang; Yong-Hui Shi; Guo-Wei Le; Effect of dietary oxidized tyrosine products on insulin secretion via the oxidative stress-induced mitochondria damage in mice pancreas. RSC Advances 2017, 7, 26809-26826, 10.1039/C7RA02945D.
- Xiaoping Chen; Wenying Yang; Branched‐chain amino acids and the association with type 2 diabetes. Journal of Diabetes Investigation 2015, 6, 369-370, 10.1111/jdi.12345.
- Petri Wiklund; Xiaobo Zhang; Satu Pekkala; Reija Autio; Lingjia Kong; Yifan Yang; Sirkka Keinänen-Kiukaanniemi; Markku Alen; Sulin Cheng; Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Scientific Reports 2016, 6, 24540-24540, 10.1038/srep24540.
- Chisato Nagata; Kozue Nakamura; Keiko Wada; Michiko Tsuji; Yuya Tamai; Toshiaki Kawachi; Branched-chain Amino Acid Intake and the Risk of Diabetes in a Japanese Community: The Takayama Study. American Journal of Epidemiology 2013, 178, 1226-1232, 10.1093/aje/kwt112.
- Xue Zhao; Qing Han; Yujia Liu; Chenglin Sun; Xiaokun Gang; Guixia Wang; The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. Journal of Diabetes Research 2016, 2016, 1-12, 10.1155/2016/2794591.
- Chizumi Yamada; Masumi Kondo; Noriaki Kishimoto; Takeo Shibata; Yoko Nagai; Tadashi Imanishi; Takashige Oroguchi; Naoaki Ishii; Yasuhiro Nishizaki; Association between insulin resistance and plasma amino acid profile in non‐diabetic J apanese subjects. Journal of Diabetes Investigation 2015, 6, 408-415, 10.1111/jdi.12323.
- C. Gar; M. Rottenkolber; Cornelia Prehn; Jerzy Adamski; J. Seissler; A. Lechner; Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Critical Reviews in Clinical Laboratory Sciences 2017, 55, 21-32, 10.1080/10408363.2017.1414143.
- Farah Alghamdi; Merry Guo; Samar Abdulkhalek; Nicola Crawford; Schammim Ray Amith; Myron R. Szewczuk; A novel insulin receptor-signaling platform and its link to insulin resistance and type 2 diabetes. Cellular Signalling 2014, 26, 1355-1368, 10.1016/j.cellsig.2014.02.015.
- Iryna Liauchonak; Bessi Qorri; Fady Dawoud; Yatin Riat; Myron R. Szewczuk; Non-Nutritive Sweeteners and Their Implications on the Development of Metabolic Syndrome. Nutrients 2019, 11, 644, 10.3390/nu11030644.
- Iryna Liauchonak; Fady Dawoud; Yatin Riat; Bessi Qorri; Manpreet Sambi; Justin Jain; Regina-Veronicka Kalaydina; Nicole Mendonza; Komal Bajwa; Myron R. Szewczuk; et al. The Biased G-Protein-Coupled Receptor Agonism Bridges the Gap between the Insulin Receptor and the Metabolic Syndrome. International Journal of Molecular Sciences 2018, 19, 575, 10.3390/ijms19020575.
- Rebecca Chaplin; Lyna Thach; Morley D. Hollenberg; Yingnan Cao; Peter Little; Danielle Kamato; Insights into cellular signalling by G protein coupled receptor transactivation of cell surface protein kinase receptors. Journal of Cell Communication and Signaling 2017, 11, 117-125, 10.1007/s12079-017-0375-9.
- Jérémie Boucher; Andre Kleinridders; C. Ronald Kahn; Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harbor Perspectives in Biology 2014, 6, a009191-a009191, 10.1101/cshperspect.a009191.
- Huaizhu Wu; Christie M. Ballantyne; Metabolic Inflammation and Insulin Resistance in Obesity. Circulation Research 2020, 126, 1549-1564, 10.1161/circresaha.119.315896.
- Huaizhu Wu; Christie M. Ballantyne; Skeletal muscle inflammation and insulin resistance in obesity. Journal of Clinical Investigation 2017, 127, 43-54, 10.1172/jci88880.
- Ambreen Asghar; Nadeem Sheikh; Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cellular Immunology 2017, 315, 18-26, 10.1016/j.cellimm.2017.03.001.
- Pavan K. Battiprolu; Thomas G. Gillette; Zhao Wang; Sergio Lavandero; Joseph A. Hill; Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discovery Today: Disease Mechanisms 2010, 7, e135-e143, 10.1016/j.ddmec.2010.08.001.
- Chang-Hua Zhang; Bu-Gao Zhou; Jun-Qing Sheng; Yang Chen; Ying-Qian Cao; Chen Chen; Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies. Pharmacological Research 2020, 159, 104984, 10.1016/j.phrs.2020.104984.
- Domenico Sergi; Nenad Naumovski Naumovski; Leonie Heilbronn Kaye Heilbronn; Mahinda Abeywardena; Nathan O’Callaghan; Lillà Lionetti; Natalie Luscombe-Marsh Luscombe-Marsh; Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Frontiers in Physiology 2019, 10, 532, 10.3389/fphys.2019.00532.
- Ansab Akhtar; Sangeeta Pilkhwal Sah; Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer's disease. Neurochemistry International 2020, 135, 104707, 10.1016/j.neuint.2020.104707.
- Evelien P. J. G. Neis; Cornelis H. C. DeJong; Sander S. Rensen; The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930-2946, 10.3390/nu7042930.
- S. Haufe; S. Engeli; J. Kaminski; H. Witt; D. Rein; B. Kamlage; W. Utz; J.C. Fuhrmann; V. Haas; A. Mähler; et al. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutrition, Metabolism and Cardiovascular Diseases 2017, 27, 858-864, 10.1016/j.numecd.2017.07.001.
- Xiaoyu Liao; Bingyao Liu; Hua Qu; Linlin Zhang; Yongling Lu; Yong Xu; Zhaohui Lyu; Hongting Zheng; A High Level of Circulating Valine Is a Biomarker for Type 2 Diabetes and Associated with the Hypoglycemic Effect of Sitagliptin. Mediators of Inflammation 2019, 2019, 1-7, 10.1155/2019/8247019.
- Christian Hellmuth; Franca Fabiana Kirchberg; Nina Lass; Ulrike Harder; Wolfgang Peissner; Berthold Koletzko; Thomas Reinehr; Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. Journal of Diabetes Research 2015, 2016, 1-10, 10.1155/2016/2108909.
- Ibiye Owei; Nkiru Umekwe; Frankie Stentz; Jim Wan; Samuel Dagogo-Jack; Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism 2019, 98, 76-83, 10.1016/j.metabol.2019.06.011.
- Celeste C. Thomas; Louis H. Philipson; Update on Diabetes Classification. Medical Clinics of North America 2015, 99, 1-16, 10.1016/j.mcna.2014.08.015.
- Hidetoshi Komatsu; Mamoru Fukuchi; Yugo Habata; Potential Utility of Biased GPCR Signaling for Treatment of Psychiatric Disorders. International Journal of Molecular Sciences 2019, 20, 3207, 10.3390/ijms20133207.
- Guido Sebastiani; Elena Ceccarelli; Maria Grazia Castagna; Francesco Dotta; G-protein-coupled receptors (GPCRs) in the treatment of diabetes: Current view and future perspectives. Best Practice & Research Clinical Endocrinology & Metabolism 2018, 32, 201-213, 10.1016/j.beem.2018.02.005.
- Eric M. Wauson; Andrés Lorente-Rodríguez; Melanie H. Cobb; Minireview: Nutrient Sensing by G Protein-Coupled Receptors. Molecular Endocrinology 2013, 27, 1188-1197, 10.1210/me.2013-1100.
- Lei Chun; Wen-Hua Zhang; Jian-Feng Liu; Structure and ligand recognition of class C GPCRs. Acta Pharmacologica Sinica 2012, 33, 312-323, 10.1038/aps.2011.186.
- Michiko Murakoshi; Harumi Kuwabara; Miyuki Nagasaki; Yu Mei Xiong; Jeff D. Reagan; Hiroaki Maeda; Futoshi Nara; Discovery and pharmacological effects of a novel GPR142 antagonist. Journal of Receptors and Signal Transduction 2016, 37, 290-296, 10.1080/10799893.2016.1247861.
- Jingru Wang; Juan J. Carrillo; Hua V. Lin; GPR142 Agonists Stimulate Glucose-Dependent Insulin Secretion via Gq-Dependent Signaling. PLOS ONE 2016, 11, e0154452, 10.1371/journal.pone.0154452.
- Darren Riddy; Philippe Delerive; Roger Summers; Patrick Sexton; Christopher J. Langmead; G Protein–Coupled Receptors Targeting Insulin Resistance, Obesity, and Type 2 Diabetes Mellitus. Pharmacological Reviews 2017, 70, 39-67, 10.1124/pr.117.014373.
- Jeffrey S. Scow; Ali Tavakkolizadeh; Ye Zheng; Michael G. Sarr; Acute “adaptation” by the small intestinal enterocyte: A posttranscriptional mechanism involving apical translocation of nutrient transporters. Surgery 2011, 149, 601-605, 10.1016/j.surg.2011.02.001.
- Heidy Sierra; Miguel Cordova; Chih-Shan Jason Chen; Milind Rajadhyaksha; Confocal Imaging–Guided Laser Ablation of Basal Cell Carcinomas: An Ex Vivo Study. Journal of Investigative Dermatology 2015, 135, 612-615, 10.1038/jid.2014.371.
- Shingo Takai; Yu Watanabe; Keisuke Sanematsu; Ryusuke Yoshida; Robert F. Margolskee; Peihua Jiang; Ikiru Atsuta; Kiyoshi Koyano; Yuzo Ninomiya; Noriatsu Shigemura; et al. Effects of insulin signaling on mouse taste cell proliferation. PLOS ONE 2019, 14, e0225190, 10.1371/journal.pone.0225190.
- Eric M. Wauson; Elma Zaganjor; A-Young Lee; Marcy L. Guerra; Anwesha B. Ghosh; Angie L. Bookout; Chris P. Chambers; Arif Jivan; Kathleen McGlynn; Michele R. Hutchison; et al. The G Protein-Coupled Taste Receptor T1R1/T1R3 Regulates mTORC1 and Autophagy. Molecular Cell 2012, 47, 851-862, 10.1016/j.molcel.2012.08.001.
- Polina L. Yarova; Alecia L. Stewart; Venkatachalem Sathish; Rodney D. Britt Jr.; Michael A. Thompson; Alexander Lowe; Michelle Freeman; Bharathi Aravamudan; Hirohito Kita; Sarah Brennan; et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Science Translational Medicine 2015, 7, 284ra60-284ra60, 10.1126/scitranslmed.aaa0282.
- A D Conigrave; A H Franks; E M Brown; S J Quinn; L-Amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism?. European Journal of Clinical Nutrition 2002, 56, 1072-1080, 10.1038/sj.ejcn.1601463.
- Antonello E. Rigamonti; Roberto Leoncini; Alessandra De Col; Sofia Tamini; Sabrina Cicolini; Laura Abbruzzese; Silvano G. Cella; Alessandro Sartorio; The Appetite−Suppressant and GLP-1-Stimulating Effects of Whey Proteins in Obese Subjects are Associated with Increased Circulating Levels of Specific Amino Acids. Nutrients 2020, 12, 775, 10.3390/nu12030775.
- Jan Gojda; Radka Straková; Andrea Plíhalová; Petr Tuma; Jana Potočková; Jan Polák; Michal Anděl; Increased Incretin But Not Insulin Response after Oral versus Intravenous Branched Chain Amino Acids. Annals of Nutrition and Metabolism 2017, 70, 293-302, 10.1159/000475604.
