Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
The increasing extension in life expectancy of humans in advanced countries matches a higher prevalence of a number of lifestyle- and age-associated pathological conditions such as cancer, systemic and neurodegenerative diseases, amyloid diseases, particularly Alzheimer’s disease (AD) and Parkinson’s (PD) disease, cardiovascular diseases (CVDs) and metabolic diseases including metabolic syndrome (MetS); the latter includes, in addition to type 2 diabetes mellitus (T2DM), CVDs and non-alcoholic hepatitis. These pathologies are characterized by several common features, including, among others, derangement of proteostasis, metabolism regulation and the redox equilibrium and a remarkable inflammatory response that heavily impairs the biochemical and functional features of the affected tissues. Moreover, at present, these pathologies, particularly amyloid diseases, lack effective therapies; it is then evident that, in the light of the latter aspect, prevention appears as the best tool to reduce the risk, or the time of appearance, of these pathological conditions. Accordingly, medical research has progressively focused on the importance of lifestyle. Physical exercise, mental activity and diet, intended as the complex of foods and nutrients taken daily by a person, are three pillars of a healthy lifestyle.
The Mediterranean diet (MD) has been the subject of a huge amount of studies on its properties to prevent different chronic-degenerative diseases from the first evidence in the early 1960s suggesting an association between the alimentation of Mediterranean people and their low cardiovascular mortality [
1]. An increasing number of epidemiological and observational studies confirm that the Mediterranean diet (MD) is associated with well aging, a condition where the prevalence of diseases including MetS, CVDs, cancer and cognitive decline appears significantly reduced [
2]. The MD can be considered as the heritage of a complex socio-economic development of the Mediterranean populations over the past centuries, and includes practices resulting from agricultural, social, territorial and environmental factors intimately associated with the culture and lifestyle of these populations. Recently, modifications of the classical MD have been proposed by the Mediterranean Diet Foundation Expert Group [
3]. The new MD pyramid, in addition to the correct presence of characteristic foods (low meat/fish, high fruit, vegetables and carbohydrates, moderate amounts of red wine, the intake of olive oil as the main lipid source, moderate caloric intake), also emphasizes the importance of other lifestyle-associated elements, such as moderation, seasonality, adequate rest, conviviality, and physical exercise. The new pyramid also reflects the changes that the MD is undergoing, at the present, within the Mediterranean societies in relation to various geographical, cultural and socio-economic contexts. The high value of the MD and its associated lifestyle was recognized in 2010 by UNESCO, who inscribed the MD in the list of the Intangible Cultural Heritage of Humanity (
https://ich.unesco.org/en/RL/mediterranean-diet-00884, accessed on 2013).
An important feature of the MD is the daily consumption of a vast array of phytonutrients including vitamins and plant phenols, which provides its similarities with the Asian diet. In particular, plant polyphenols interfere with multiple signaling pathways involved in protein homeostasis, in the inflammatory response, and in the regulation of both metabolism and the antioxidant defenses [
4,
5,
6], often recalling a caloric restriction (CR) regimen. These features positively affecti among others, whole body metabolism, mitochondrial turnover, oxidative stress and the inflammatory and neuroinflammatory response, where autophagy plays an important role [
7,
8]. Polyphenols can reach these effects by counteracting, at the molecular level, signaling pathways responsible for the cascade reactions involved in aging [
9,
10]. Overall, present data support the idea that different plant polyphenols, including those from the olive tree, are able to mimic CR effects and to modulate the expression of pro- and anti-apoptotic factors, also through epigenetic modifications [
11], thus affecting the same, or very similar, cellular targets. Accordingly, plant polyphenols can be proposed as a useful tool for the prevention and/or treatment of aging-associated diseases connected with chronic inflammation or transcriptional, redox or metabolic derangement [
12].
An increasing number of preclinical studies, population studies and clinical trials suggest that adherence to the MD, with particular emphasis on its content of plant polyphenols, often referred to as biophenols, reduces metabolic pathologies and aging-associated deterioration, where derangement of redox homeostasis and an excessive inflammatory response often play pivotal roles. Biophenols are found in many foods of plant origin that play pivotal roles in the MD, including red wine, extra virgin olive oil (EVOO), green tea, spices, berries and aromatic herbs. The content of polyphenols in these foods and their bioavailability are quite low; however, the daily consumption, throughout one’s lifetime, of these foods ensures a reduced, yet continuous, intake of polyphenols, providing a rationale for the association between the dietary content of the latter and a significant reduction in the incidence of aging-associated pathologies reported by many population/epidemiological studies and clinical trials [
13,
14].
A wealth of recent studies has highlighted the fact that, in several aging-associated pathologies such as amyloid diseases, CVDs and MetS, plant polyphenols do not simply interfere with a single step of disease pathogenesis (protein/peptide aggregation, the inflammatory response, the redox/metabolic equilibrium, the proteostasis balance); rather, their positive biological and functional outcomes result from multi-target effects leading to the restoration of altered homeostatic systems in cells and tissues. In addition, the chemical similarities of these structurally distinct molecules can explain why they can induce similar effects. Among others, the importance of natural polyphenols for health has been associated with their remarkable antioxidant power elicited through the modulation of oxidative pathways. The latter can result from interference with enzymes, proteins, receptors, transcription factors and several signaling pathways [
4,
15]. The ability of plant polyphenols to interfere with biochemical homeostasis has also been taken into consideration [
14], and epigenetic modifications of chromatin have been reported to be also involved in these effects [
16,
17]. Actually, recent research is providing increasing information on the biochemical, cellular and epigenetic modifications induced by several plant polyphenols and the resulting modulation of the homeostasis of key cellular processes such as metabolism, energy balance, redox equilibrium, proteostasis, cell signaling, the inflammatory response, and the control of oxidative stress and of gene expression. The knowledge stemming from these data will allow us to better understand the beneficial effects, for human wellness, of the MD, the importance of its content in plant polyphenols and the role of the latter in disease prevention and, possibly, therapy.
The rising interest in natural polyphenols has resulted in a large number of studies on their medicinal efficacy, carried out not only in cultured cells but also in model organisms and in humans. More recently, an increasing number of studies have also appeared on the biochemical and biological effects of olive polyphenols. The polyphenols elaborated by the olive tree (
Olea europaea) are present prevalently in the leaves and drupes of the tree and are important as phytoalexins, molecules that the plant elaborates for defense against invasions by microbes and fungi and to discourage leaf-eating insects. EVOO contains over 30 phenolic compounds, including the most represented oleuropein, both in the glycated and in the aglycone (OLE) form, verbascoside, oleocanthal, hydroxytyrosol (HT), tyrosol, and others (see next section). The healthy value of EVOO and olive leaf extracts has been recognized for a long time and scientifically investigated in the last couple of centuries. More recent studies have focused on the biological properties of these molecules, including the antimicrobial, hypoglycemic, vasodilator, antihypertensive, antioxidant and anti-inflammatory ones, whose clinical importance was first reported in 1950 [
18]. These properties have led to the inclusion of the alcoholic extract (80%) of olive leaves containing, in addition to minor components, OLE, HT, caffeic acid, tyrosol, apigenin and verbascoside in the European Pharmacopoeia (Ph. Eur.) [
19,
20].
The molecular determinants of the protection by olive polyphenols against several aging-associated and chronic degenerative conditions, including T2DM [
21,
22,
23] and non-alcoholic fatty liver disease [
24,
25,
26,
27,
28], have been extensively investigated in the last 20 years. OLE, HT and other olive polyphenols protect cells against oxidative damage resulting from redox dyshomeostasis [
29,
30] and an excessive inflammatory response [
31], among the main determinants of age-related pathologies such as cancer, T2DM, MetS, osteoporosis and neurological diseases [
27,
32]. Most of these effects have been associated with the ability of polyphenols to control cell signaling and pathways, to modulate the activity of transcription factors, and to affect gene expression; these nutrigenomic properties of EVOO polyphenols have been recently reviewed [
33,
34]. Finally, population studies have provided evidence of a significant association between MD, EVOO consumption, and reduced risk of both CVD [
35] and cognitive decline [
2]. A recent review of the scientific literature focused on clinical trials and population studies has confirmed that the MD and the fortification of the foods with olive leaf extracts protect significantly against several aging-associated degenerative diseases and cancer [
36,
37,
38]. Accordingly, plant polyphenols are increasingly taken into consideration, as such or their molecular scaffolds, as the starting component to develop new drugs especially designed to combat several chronic degenerative pathologies, including aging-associated neurodegeneration [
36,
39].
2. Olive Polyphenols: A Group of Molecules with Shared Chemical and Biological Properties: Structure, Abundance and Bioavailability
Biophenols are a family of over 8000 polyphenolic structures (those presently described) found in almost all plant families mainly as secondary metabolites, including several hundred isolated from edible plants [
36,
40]. These molecules include non-flavonoids or flavonoids; the latter are further classified as flavonols, flavononols, flavones, anthocyanins, procyanidins, phenolic acids, stilbenes and tannins on the basis of the number of hydroxyls in the molecule and the type and the position of other substituents [
41]. The plant sources of plant polyphenols are, among others, bark, leaves, fruits, spices, berries, vegetables, roots, nuts and seeds, herbs, and whole grain products, from which they are transferred in processed foods of plant origin, including EVOO, red wine, green tea, coffee and turmeric. These compounds are characterized by a broad spectrum of biological activities and exert positive effects in a large number of human diseases, including cancer, CVDs, T2DM and neurodegenerative conditions, with molecular mechanisms often related to their antioxidant activity. In the case of EVOO, its healthy properties have been associated with its peculiar chemical composition [
42]. EVOO contains both major components (triglycerides and other fatty acid derivatives where mainly monounsaturated fatty acids, in particular oleic acid, are present) and minor components (over 230 different chemicals including aliphatic and triterpenic alcohols, phytosterols, hydrocarbons, tocopherols, and polyphenols) [
43]. In the past, the health effects of EVOO were attributed mainly to the presence of oleic acid; however, more recently, attention has been focused on phenolics, a class of bioactive compounds including phenolic acids, phenolic alcohols, flavonoids, secoiridoids and lignans [
44].
In particular, olive tree polyphenols include flavonols, lignans and glycosides. Olive glycosides are iridoids, geraniol-derived monoterpenes, whose chemical structure results from a cyclopentane ring fused to a six-member heterocycle with an oxygen atom. In particular, the bicyclic H-5/H-9β, β-
cis-fused cyclopentanepyran ring system is the most common structural feature and the basic skeletal ring of iridoids. Cleavage of the cyclopentane ring of iridoids produces seco-iridoids, while cleavage of the pyran ring produces iridoid derivatives [
45]. Iridoids and secoiridoids, mainly in the glycated form, are found in many medicinal plants belonging to the subclass Asteridae that includes several plant families, particularly Oleaceae.
The polyphenols produced by the olive tree are found in the lipid fraction and in the water fraction (dispersed as minute droplets) of olive oil mainly in the glucose-free form (aglycones), resulting from deglycosylation by plant glycosidases during olive squeezing. The most abundant secoiridoid in olive oil is 3,4-dihydroxyphenylethanol-elenolic acid (3,4-DHPEA-EA), whose glucose-bound form is commonly known as oleuropein; the latter is the main cause of the bitter taste of olive leaves and drupes. Other secoiridoids include oleuropein derivatives, both in the glucose-bound form or as aglycones, such as the dialdehydic form of decarboxymethyl elenolic acid bound to either HT (3,4-dihydroxyphenylethanol-elenolic acid dialdehyde, 3,4-DHPEA-EDA, also known as oleacein) or to tyrosol (p-hydroxyphenylethanol-elenolic acid dialdehyde, p-HPEA-EDA, also known as oleocanthal, or ligstroside aglycone [
46,
47] (
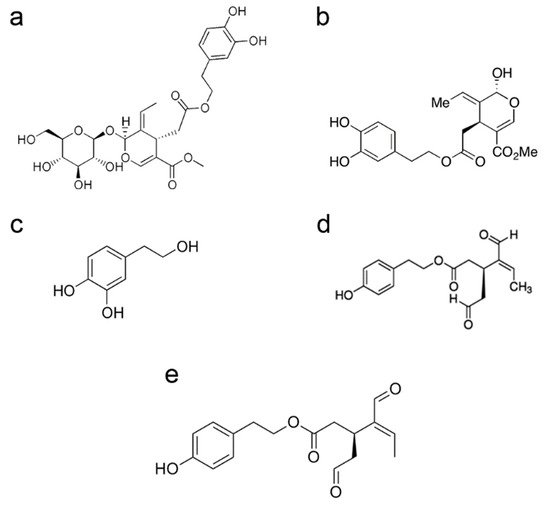
Figure 1). Oleocanthal produces the burning sensation in the back of the throat that accompanies the consumption of freshly squeezed EVOO. Olive oil also has a rich composition in simple phenols; these include tyrosol (p-hydroxyphenylethanol, p-HPEA) and hydroxytyrosol (3,4-dihydroxyphenylethanol, 3,4-DHPEA, DOPET), two phenolic alcohols mostly derived from their secoiridoid precursors. Olive polyphenols also include verbascoside, the caffeoylrhamnosylglucoside of HT, 1-acetoxypinoresinol and pinoresinol (two lignans).
Figure 1. Chemical structure of oleuropein (a), oleuropein aglycone (b), hydroxytyrosol (c), oleacein (d), oleocanthal (e).
Olive polyphenols are considered to be responsible for some of the recognized pharmacological properties of the olive tree (anti-atherogenic, antihepatotoxic, hypoglycemic, anti-inflammatory, antitumoral, antiviral, analgesic, purgative and immunomodulatory activities) [
28,
48,
49], together with the protection against aging-associated neurodegeneration [
29]. For these reasons, the EVOO quality depends not only on the content in free fatty acids resulting from triacylglycerol breakdown (acidity), but also on its content in polyphenols, the molecules responsible for its taste and for many of its healthy properties. Several factors affect the content of polyphenols in olive oil; these include olive cultivar, environmental cues (altitude, meteorological factors and irrigation), cultivation practices, and ripening stage of the fruits [
50], together with extraction techniques, systems to separate oil from olive pastes. The conditions of storage (temperature, time) are also of importance, affecting the rate of oxidation/photooxidation reactions and the deposition of suspended water particles rich in polyphenols [
51]. Under optimal conditions, the content of polyphenols in EVOO can exceed 60 mg/100 g.
The normal daily dietary intake of plant polyphenols is in the 0.1–1.0 g range; however, the bioavailability of these molecules, including the olive ones, in humans is poor due to reduced intestinal absorption and fast biotransformation that favors their urinary excretion. In addition, in the case of the brain, the circulating polyphenols must also cross the blood–brain barrier before reaching the parenchyma. With few exceptions, polyphenol aglycones can be partially absorbed in the small intestine by passive diffusion [
52] much better than their glycated counterparts [
53], although important amounts proceed to the large intestine to be eliminated [
54]. A review of many studies on polyphenol bioavailability reported a 0 to 4.0 µmol/L plasma concentration of total metabolites produced from the oral administration of 50 mg aglycone equivalents of a polyphenol [
55]. After intestinal absorption and passage to the lymph, most polyphenols undergo phase I and phase II metabolism, with substantial biotransformation and production of methylated, sulphated, hydroxylated, thiol-conjugated and glucuronide derivatives and degradation products [
56]. These modifications alter the chemical properties of plant polyphenols, favor their excretion and, possibly, provide them new biological activities [
57]. The importance of the colonic microflora for polyphenol bioavailability, due to its ability to metabolize and chemically modify polyphenols, has been reported recently [
55]. Anyway, recent studies indicate that plant, including olive, polyphenols are absorbed in discrete amounts from the intestine and rapidly distributed through the blood flow to the whole organism, including the brain, both in rats [
58,
59] and in humans [
60,
61]. Plant polyphenols do interact with, and cross, synthetic and cell membranes. The interaction of oleuropein aglycone with synthetic phospholipid membranes favored by the presence of anionic lipids has been reported in a very recent study [
62]. Another study reported that several polyphenols (the olive ones were not included) protected the mitochondria against membrane permeabilization by amyloid oligomers, suggesting some interference with oligomers’ interaction with the membrane [
63]. Finally, oleuropein aglycone (OLE) was the main polyphenol found in breast cancer cells treated with an olive leaf extract in a recent metabolite-profiling study, suggesting its ability to cross the plasma membrane of these cells [
64].
Due to the rising interest in natural phenols as possible new drugs, strategies to improve their bioavailability are under study, with encapsulation being probably the most actively investigated, in some cases with encouraging results [
65,
66]. Most of these molecular tools have not been tested in clinical trials, yet this strategy appears promising to improve the efficacy of natural phenols as drugs while reducing the amount of the administered dose. Actually, accurate studies on the effective dose of olive polyphenols to be administered daily to humans to obtain significant protection are still lacking; at any rate, the amount of OLE and other plant polyphenols taken daily in foods appears not adequate to ensure a dose suitable to produce short-term acute effects. However, clinical and experimental evidence indicates that a continuous consumption of moderate amounts of these molecules can be effective in the long term; this can also result in the accumulation in body tissues of these lipophilic molecules, leading to a low-intensity continuous stimulus of cell defenses against amyloid deposition, protein and metabolism dyshomeostasis, oxidative stress and other alterations underlying age-associated pathologies. These effects, although not proven experimentally, could, at least in part, explain the healthy properties of the MD. Nevertheless, the intake of moderate amounts of olive, and other plant, polyphenols provided by a typical MD supports the usefulness of the integration of polyphenol-enriched olive leaf extracts and other polyphenol-enriched nutraceuticals that can intensify, in the short term, the beneficial effects of these molecules.
3. Antioxidant and Anti-Inflammatory Properties of Olive Polyphenols in Animal Models
It is widely recognized that oxidative and nitrosative stress as well as inflammation are the major abnormalities underlying neurodegeneration and that antioxidant molecules, such as olive oil polyphenols, restore neuronal function through the amelioration of the redox status. Some beneficial effects of the MD have been associated with the consumption of EVOO polyphenols; these include antioxidant, hypoglycemic, antimicrobial, antiviral, antitumor, cardioprotective, neuroprotective, antiaging and anti-inflammatory activities [
67,
68]. It has been reported that EVOO polyphenols are protective against cognitive impairment associated with aging and neurodegenerative diseases due to their ability to protect DNA against oxidative stress, to inhibit mitochondrial dysfunction and to attenuate lipid peroxidation by scavenging free radicals, thus sustaining endogenous antioxidant stability [
69,
70]. They are also able to inhibit amyloid β (Aβ) and τ protein aggregation and toxicity, the main causes of the neurodegenerative cascade in AD [
39,
71,
72]. EVOO polyphenols participate in the redox balance of the cell as antioxidants and as mild pro-oxidants, with ensuing upregulation of the antioxidant defenses of the cell. Accordingly, they can be considered as hormetic factors. For instance, in the presence of peroxidases, HT can undergo a redox cycling that generates superoxide [
70], and tyrosol also increases
C. elegans lifespan by activating the heat shock response [
71]. It was reported that HT reduces brain mitochondrial oxidative stress and neuroinflammation in AD-prone transgenic mice by the induction of Nrf2-dependent gene expression [
72]. The eight-week administration of oleuropein (60 mg/kg/day) improved mitochondrial function and reduced oxidative stress by activating the Nrf2 pathway in SHR rats [
73]. Furthermore, tyrosol (240 mg/kg) was found to be protective against LPS-induced acute lung injury
through the inhibition of NF-κB and the activation of AP-1 and of the Nrf-2 pathway [
74]. EVOO polyphenols also enhance Nrf-2 activation at the hepatic level and the ensuing release of antioxidant enzymes [
75]. Nrf2 is considered the principal regulator of redox homeostasis and its activation inhibits pro-inflammatory mediators such as cytokines, COX-2 and iNOS [
76]. EVOO polyphenols limit inflammation by reducing the expression/activity of the transcription factors NF-κB and AP-1 [
77] thanks to their free radical scavenging and radical chain breaking capacity and to the reduced formation of ROS and RNS. Moreover, HT inhibits the development of the inflammatory cascade following LPS and carrageenan injection through downregulation of the levels of pro-inflammatory cytokines (TNF-α and IL-1β), COX2, iNOS, NO, PGE2 and NF-kB and reducing DNA damage [
78,
79,
80]. It was reported that the co-injection of OLE (450 µM) with Aβ42 (50 µM) into the nucleus basalis magnocellularis (NBM) of adult rats interfered with Aβ aggregation and significantly counteracted Aβ toxicity against choline acetyltransferase-positive neurons of the NBM and reduced astrocyte and microglia activation [
81]. Another study reported that OLE protects transgenic
C. elegans strains, constitutively expressing Aβ3-42, by reducing Aβ plaque load and motor deficits [
82]. Interestingly, significant anti aggregation and neuroprotective effects of a diet supplemented with OLE, HT or a mix of polyphenols from olive mill wastewater were reported in the TgCRND8 mouse model of Aβ deposition. In these transgenic mice, a significant improvement in cognitive functions and a significant reduction in Aβ plaque number, size, and compactness were found in 3- and 6-month-old mice (at the early and intermediate stage of Aβ deposition, respectively) fed for 8 weeks with the OLE-supplemented diet [
83,
84,
85]. A significant improvement in synaptic function and a significant reduction in the number, size and compactness of both Aβ42 and its 3-42 pyroglutamylated derivative (pE3-Aβ) deposits occurred even when the treatment was started at 10 months, when these mice display increased brain deposits of Aβ and, in particular, of pE3-Aβ in the cortex and hippocampal areas. These data indicate that oral diet supplementation with OLE not only results in the prevention of amyloid deposition but also in the disaggregation of preformed plaques and in a reduction in pE3-Aβ generation [
85]. The effect of OLE against Aβ peptide aggregation was dose-dependent and could be reproduced by diet supplementation with a mix of polyphenols from olive mill wastewater or by HT administered at the same dose as that of pure OLE [
84,
86]. Interestingly, the treatment with OLE (50 mg/kg of diet for 8 weeks) astonishingly activated neuronal autophagy even in TgCRND8 mice at an advanced stage of pathology. In these animals, histone 3 acetylation on lysine 9 (H3K9) and histone 4 acetylation on lysine 5 (H4K5) were increased in the cortex and the hippocampus; such an increase matched both a decrease in HDAC2 expression and a significant improvement in synaptic function [
85].
It is known that abnormal acetylation takes place in memory and learning disorders such as AD, where a significant increase in HDAC2 inhibits gene expression at specific loci, such as those involving autophagy markers [
87]. In addition to the induction of an intense and functional autophagic response in the cortex, other relevant biological effects of OLE were uncovered in the TgCRND8 model; these include increased microglia migration to the plaques for phagocytosis, enhanced hippocampal neurogenesis and reduced astrocyte reaction [
83,
88]. OLE induced autophagy through the increase in cytosolic levels of Ca
2+ and the subsequent activation of the enzyme complex AMPK by Ca
2+/Calmodulin Protein Kinase Kinase β (CaMKKβ) and the ensuing increase in phosphorylation of mammalian target of rapamycin (mTOR) with mTOR inhibition [
89]. These data support the idea that autophagy activation by OLE and other olive polyphenols proceeds via modulation of the AMPK–mTOR axis, similarly to data reported for other plant polyphenols [
90]. TgCRND8 mice fed with a diet supplemented with OLE or HT (50 mg/kg of food) exhibited increased levels of Beclin-1 and LC3 autophagic markers in the soma and dendrites of neurons of the somatosensory/parietal and entorhinal/piriform cerebral cortex, together with improved autophagosome/lysosome fusion [
83,
86]. Furthermore, the significant accumulation of PAR polymers and the increase in PARP1 expression found in the cortex at the early (3.5 months) and intermediate (6 months) stage of Aβ deposition in the TgCRND8 mice were rescued to control values by OLE supplementation. OLE-induced reduction in PARP1 activation was paralleled by the overexpression of SIRT1, and by a decrease in the pro-inflammatory NF-κB and the pro-apoptotic p53 marker [
88].
The ability of EVOO polyphenols to modulate the action of NF-kB was observed both in vitro and in vivo in different tissues. In vivo, HT attenuated apoptosis in rat brain cells by modulating the levels of caspase-3 and NF-kB p65 subunit [
91]; in high-fat diet (HFD)-fed C57BL/6 J male mice, daily doses of HT (5.0 mg/kg) attenuated the increment of NF-κB and SREBP 1c, and increased the activity of Nrf2 and PPAR-γ in the liver [
92]. In female BALB/c mice, an EVOO-supplemented diet was protective in the management of induced systemic lupus erythematosus disease, likely through the inhibition of the MAPK, JAK/STAT, and NF-κB pathways in splenocytes [
93]. One of the most studied upstream constituents of the NF-κB signaling pathway is the activation of the mitogen-activated protein kinases (MAPKs) [
94]. In the TgCRND8 mice, an HT-supplemented diet modulated MAPK signaling by activating ERK and downregulating SAPK/JNK expression, a mechanism that may underlie memory improvements in these mice [
86]. These data agree with other findings suggesting an involvement of ERK stimulation in memory formation and synaptic plasticity. In the C57BL/mouse model of AD, the administration of HT and its acetylated derivative significantly improved spatial memory deficits induced by the intracerebral injection of Aβ42 plus ibotenic acid. The latter affected the Bcl-2/Bad levels, activated caspase/cytochrome-dependent apoptosis, and downregulated pro-survival genes also involved in memory functions (Sirt-1, CREB, and CTREB target genes), whereas HT administration alleviated these alterations [
95]. Taken together, these data suggest that OLE and/or its metabolite, HT, can be effective to combat cellular alterations underlying AD symptoms in the absence of undesirable side effects.
Finally, HT was shown to inhibit the toxicity associated with α-synuclein aggregation in PD [
96]; HT and OLE improved spatial working memory and energetic metabolism in the brain of aged mice [
97]; and HT decreased oxidative stress in the brain of
db/db mice, a widely used human T2DM animal model, by improving mitochondrial function and inducing phase II antioxidative enzymes through the activation of the Nrf2–ARE pathway [
98].
To date, less data have been reported for oleocanthal. Recently, in vitro and in vivo studies reported that oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism [
99,
100].
4. Antioxidant Properties of Olive Polyphenols: Molecular Mechanisms
The overproduction of ROS correlates with lipid, protein or DNA damage involved in the onset of degenerative diseases; accordingly, cell defenses against a rise in ROS are fundamental [
101]. Antioxidants inhibit oxidation; therefore, to react to oxidative stress, organisms maintain complex systems of antioxidants, primarily glutathione (GSH). Unfortunately, only a few drugs and biological molecules, such as vitamins, have been reported to act as antioxidants, yet with possible side effects [
102,
103].
Nowadays, researchers are focusing their attention on the antioxidant properties of natural compounds, without relevant side effects. In particular, the importance of the antioxidant activity of lipophilic and hydrophilic phenols in EVOO has emerged [
104]. This fraction is physiologically produced by plants to react against the injuries produced by various pathogens or insects [
28,
105]. The antioxidant activity of the major phenolic components of EVOO, OLE and HT is related to their relative bioavailability with an appreciable level of absorption, fundamental to exert their metabolic and pharmacokinetic properties [
49]. In molecular terms, OLE and HT, with their catecholic structure, behave as antioxidants in different ways: (i) by scavenging the peroxyl radicals and breaking peroxidative chain reactions, generating very stable resonance structures [
106]; and (ii) by acting as metal chelators, therefore, preventing the copper sulphate-induced oxidation of low-density lipoproteins [
107]. The activity of OLE and HT as metal chelators could be attributed to the ability of the hydroxyl groups to behave as electron donors and to the ensuing formation of intramolecular hydrogen bonds with free radicals [
32]. However, the scavenging activity of OLE and HT was also assessed in non-metal oxidation systems. Indeed, data obtained in vitro highlight the ability of polyphenols to reduce the inactivation of catalase (CAT) by hypochlorous acid (HOCl); this effect protects against atherosclerosis following LDL oxidation by HOCl through apoB-100 chlorination [
108]. Moreover, HT has been reported to improve the redox status of the cell by increasing the levels of GSH [
109].
Recently, the oxidative damage in age-related diseases turned out to be primarily caused by reduced levels of the transcriptional Nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2) [
110], and it was proposed as a therapeutic target for metabolic syndromes, including obesity, due to its behavior as a mediator of general adaptive responses of the cell, including proteostasis and inflammation [
111,
112]. However, the pivotal role of Nrf2 is involved in the regulation of protection against oxidation [
113]. Following Nrf2 activation and consequently its translocation to the nucleus, Nrf2 binds to antioxidant response elements (ARE); after binding, it acts on the transcriptional expression of several antioxidant enzymes, including superoxide dismutase (SOD), c-glutamylcysteine synthetase (c-GCS), glutathione S-transferase (GST) and NADPH quinone oxidoreductase-1 (NQO1) [
114].
EVOO polyphenols have been reported to interact with Nrf2 and with Nrf2-controlled enzymes. In vivo studies showed that EVOO polyphenols increased, at the mRNA level, the expression of Nrf2 and of its targets paraoxonase-2 (PON2), c-GCS, NQO1, and GST in the heart tissue of senescence-accelerated mouse-prone 8, whose diet included 10% olive oil [
115]. These effects have been ascribed to HT. Indeed, a model of metabolic alterations, the high-fat diet (HFD)-fed male mice C57BL/6J, supplemented with HT (5.0 mg/kg), displayed a reduction in oxidative stress by restoring Nrf2 and the activity of the peroxisome proliferator-activated receptor-α (PPAR-α) to normal levels [
116]. The same results were obtained when the same model was supplemented with the highest dose of HT (10–50 mg/kg/day), which also resulted in an increase in GST activity in the liver and in the muscle [
117]. Finally, spontaneously hypertensive rats fed with OLE (60 mg/kg/day) showed increased levels of Nrf2-dependent phase II enzymes, such as NQO-1 [
77]. Anyway, in spite of these and other data, the molecular mechanisms controlled by EVOO polyphenols in terms of antioxidant activity are not still clear; in fact, the reported effects were probably determined by the tissue localization of the enzyme and by the different concentrations of phenols used. Indeed, differently from previous data, in 60-day-old Wistar male rats fed with 7.5 mg/kg/day HT, oxidative stress was increased in heart tissue, probably due to the high concentration used [
118]. The latter finding is not surprising; in fact, OLE and HT exert anti-proliferative and pro-apoptotic effects on tumor cells in vitro, inducing an accumulation of hydrogen peroxide mediated by the high doses [
119,
120].
The activity of EVOO polyphenols on Nrf2 signaling and on the levels of several antioxidant enzymes, such as γ-glutamyl-cysteinyl-ligase (γ-GCL) and SOD, was also reported in in vitro experiments with LPS-treated macrophages [
121] and cancer cells [
122]. Furthermore, it is widely reported that OLE and HT act on AMPK signaling, and the latter has been considered as an attractive therapeutic target for antioxidant activity. In fact, AMPK signaling plays a fundamental role in the cell defense system against ROS by direct phosphorylation of human FoxO1 (forkhead box O1) at Thr649, with the ensuing increase in FoxO1-dependent transcription of Mn-superoxide dismutase (MN-SOD) and CAT [
123].
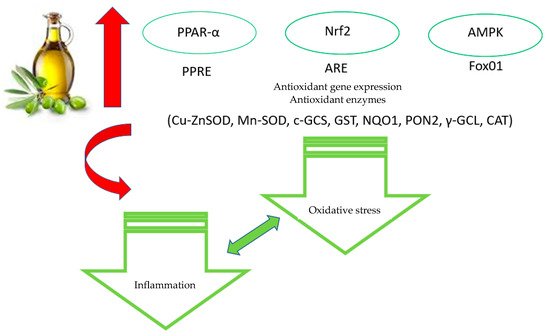
In conclusion, the data reported in the present and in the previous paragraph convincingly support the idea that EVOO polyphenols, in particular OLE and HT, exert antioxidant activity by interfering with different cellular pathways (Figure 2).
Figure 2. Main antioxidative effects of EVOO polyphenols.
This entry is adapted from the peer-reviewed paper 10.3390/antiox10071044