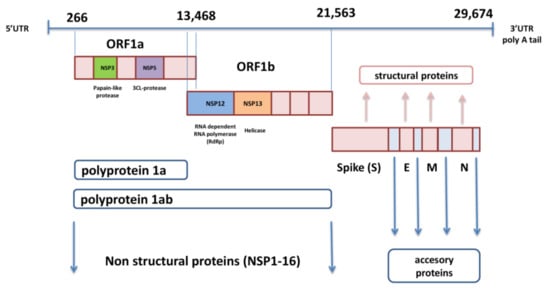
3. Implications of SARS-CoV-2 Variants in Immune Evasion
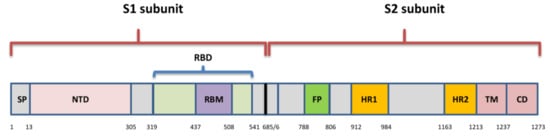
Although different lineages are defined by mutations in more than one region of the genome, the most attention is paid to nonsynonymous changes in the S gene, which can alter the spike protein and influence its role in viral entry. This role of spike has determined it as an ideal target for immune response and also made it the primary target for most currently approved vaccines. Amino acid changes have been observed across the entire spike protein, but the exact location defines the impact of each substitution. The NTD and RBD are the most diverse regions, and most mAbs against SARS-CoV-2 that have been characterized target the RBM, and some are specific for RBD core- and NTD as well [
81]. Changes in spike residues within major epitopes may reduce or ablate antibody binding and neutralization, which would lead to the diminished efficacy of antibodies, derived by natural infection or vaccination. However, changes are found to occur also within the conserved C-terminal domain of the S1 and the S2 subunit. These regions are important for conformational changes within S, which is needed for viral attachment and fusion, and may elicit still unknown neutralizing responses [
82].
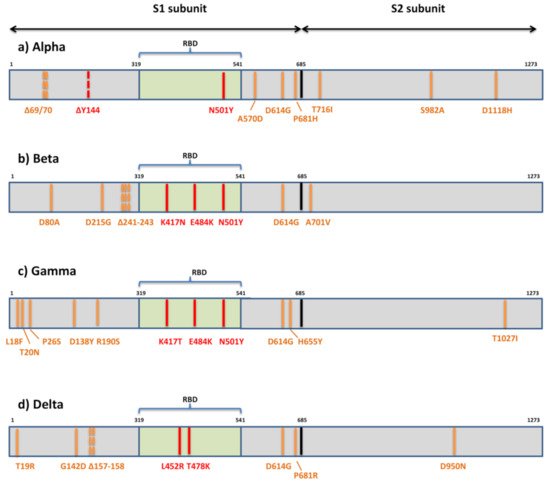
The first variant Alpha or B.1.1.7 that raised global concerns about increased transmissibility and potential immune evasion harbors seven missense mutations (N501Y, A570D, D614G, P681H, T716I, S982A, D1118H) and three deletions in spike (69/70del and 144del) (
Figure 3). The three deleted residues are located within NTD, only one mutation (N501Y) is within RBM, three are displayed in the C-terminal domain (CTD) of S1, and three are displayed within S2. Various studies have so far demonstrated the reduced potency of neutralizing antibodies against B.1.1.7 [
49,
83,
84,
85,
86,
87,
88]. These studies share the general conclusion that variant B.1.1.7 remains sensitive to neutralization, though at moderately reduced levels, by sera of convalescent individuals and recipients of several anti-COVID19 vaccines. The reduction in neutralization levels were on average 3-fold (ranging from 1.5-10-fold) for convalescent sera and ≈2-fold for sera of vaccine recipients (mRNA-, vector-, and subunit-based) [
49,
83,
84,
85,
86]. However, when various mAbs were tested against this variant, it was uniformly shown that the B.1.1.7 variant can escape neutralization mediated by a fraction of RBM-specific antibodies and by most NTD-specific antibodies [
49,
83,
88]. The proposed explanation for the more serious effect of spike mutations on neutralization by mAbs than by sera is the polyclonality of serum neutralization [
84]. It is supported by the observation that a single mutation can diminish the binding of a single mAbs but not of other antibodies in the same binding cluster. A single mutation cannot affect all antibodies in the same cluster, since it seems that each antibody has well defined and unique molecular contact with the same specific epitope. Therefore, polyclonal sera are less susceptible to changes in neutralization due to a single mutation. Polyclonal sera also contain non-neutralizing antibodies whose role is yet to be elucidated.
Figure 3. Mutational patterns of four variants of concern designated by the WHO. (a) Alpha or lineage B.1.1.7; (b) Beta or lineage B.1.351; (c) Gamma or lineage P.1; (d) Delta or lineage B.1.617.2; The residues potentially responsible for immune evasion are marked in red.
The part of the diminished neutralizing effect of antibodies against the B.1.1.7 variant can be attributed to the only RBM mutation—N501Y. This mutation, shared by three globally recognized VOCs, is thought to be the result of viral adaptive evolution [
89] and has been shown to increase affinity for ACE2 [
85,
90,
91,
92]. The enhanced binding affinity may be contributed to additional interactions with ACE2 that are allowed by 501 change—the new hydrogen bonds at residues 41 and 353 and also to a more open conformation of the RBD [
93,
94]. While some report its antigenic impact to be limited to a few mAbs with no significant effect on neutralization by convalescent or vaccinees sera [
83], others show that the increase in transmission is combined with the reduction in the neutralization potency of convalescent sera [
85]. The explanation for this lies not in the disrupted binding of antibodies to changed RBM but in competition of antibodies with ACE2 for binding to RBM. Thus, all changes in RBM that confer increased affinity for ACE2 will make the virus more difficult to neutralize.
The significant resistance of B.1.1.7 to neutralization by NTD-specific antibodies should be explained by the presence of three deleted residues in this region. The neutralization effect of these antibodies can be attributed to the role that NTD has in viral entry. While it was not yet determined for SARS-CoV-2, the NTD has a role in attachment to host cells in several CoV family members [
95]. For SARS-CoV-2, it has been proposed that NTD interacts with auxiliary receptors in cell types that do not express ACE2 (e.g., DC-SIGN/L-SIGN) [
96]. The NTD deletion H69/V70 is observed in B.1.1.7. and B.1.298 (Danish mink) but has not been associated so far with escape from NTD-specific antibodies [
88]. A combination of del H69/V70 and N501Y was shown to increase infectivity in vitro [
97]. On the other hand, deletion Y144 has been found to abrogate binding to neutralizing antibodies [
49,
52,
88,
98]. It can still not be determined whether NDT mutations are the result of immune selection or are generated as part of viral fitness improvement.
Other spike mutations of B.1.1.7 belong to the C-terminal domain of S1 and S2 and were not so far perceived to affect antibody neutralization. However, mutations within these regions might affect the conformation of RBD, attachment, and fusion, requiring further studies to determine their consequences and possible indirect effect on immune evasion. The extensively studied D614G was found to increase the ability of RBD to shift to the up position, which is necessary for interaction with ACE2 [
99]. This resulted in the increased infectivity and transmissibility observed for the D614G variant relative to the original SARS-CoV-2 strains [
100]. The P681H change is adjacent to the furin cleavage site and could potentially have an effect on S1/S2 cleavage and therefore on cell entry and infectivity.
The variant of the greatest concern in regard to immune escape, Beta or B.1.351, contains seven mutations (D80A, D215G, K417N, E484K, N501Y, D614G, A701V) and three deletions (241/242/243del) in the spike protein [
75] (
Figure 3). Two mutations (D80A, D215G) and three deleted residues are in the N-terminal domain of S1, one (A701V) is in loop 2 of S2 and 3 are at key residues in the RBD (K417N, E484K, N501Y). So far, there are multiple studies showing that B.1.351 decreases the neutralization capacity of antibodies elicited by infection with previous variants or vaccination [
48,
83,
101,
102,
103,
104,
105]. This reduction in neutralizing potential for B.1.351 is most frequently detected in individuals with low antibody levels, and it is declining more rapidly with time [
105], heightening concerns about re-infection or suboptimal protection by current vaccines. The problem in the non-vaccinated population exists because most people infected with SARS-CoV-2 develop only low to moderate titers, while higher titers are only observed in severely ill hospitalized individuals. The loss of neutralizing activity of convalescent plasma against B.1.351 ranged from 11 to 33-fold and by sera of vaccinees from 3.4 to 8.5-fold [
50,
83,
101,
103,
104,
105,
106]. In addition, the B.1.351 variant showed resistance to neutralization by most NTD-specific and a number of RBM-specific mAbs [
83,
103,
107].
The resistance to antibody neutralization of the B.1.351 variant is mainly ascribed to three mutations within RBD (K417N, E484K, N501Y). N501Y probably does not impair neutralization on its own but rather in combination with other two, which were found to partially compromise neutralization generated by previous infection or vaccination [
48,
103,
106,
107,
108]. The result of the change at position 417 is loss of the polar interaction with residue D30 on human ACE2 [
82]. However, a combination of K417N and N501Y was shown to enhance the binding with ACE2 and reduce binding with antibodies [
109]. This improvement in receptor binding is supported by the observation of this mutation in a virulent mouse adapted strain of SARS-CoV-2 [
110]. K417N was shown to be crucial to viral escape, effectively abrogating neutralization by some of the most common and potent neutralizing antibodies to SARS-CoV-2 [
103]. Contrary to this, others [
107] indicate that it may contribute to neutralization by enhancing the probability of conversion to the open conformation of the S protein, thus exposing epitopes to antibody neutralization.
Mutation E484K, which emerged independently in over 50 lineages, also corresponds with improved binding to ACE2. It enhances the binding affinity of N501Y for ACE2 still further but has been associated with immune escape from both mAbs and polyclonal sera as well [
48,
49,
83,
106,
107]. Its location is within the RBD binding cleft, and it is considered to be a dominant neutralizing epitope [
75,
108,
111]. The residue 484 can mutate into a diversity of different amino acids (E484A, E484G, E448D, and E484K) under the pressure of SARS-CoV-2 convalescent sera and exhibits resistance [
112]. It is believed that the impact of mutation 484 on immune evasion is significantly augmented by the presence of other two RBD mutations in this variant, but its impact as the single point mutation was demonstrated as well [
106,
112].
The B1.1.7 variant bearing the E484K mutation emerged and was recognized as a variant of concern in the UK and Europe, since it appears to be responsible for a significant additional loss of neutralization capacity of monoclonal and polyclonal antibodies [
49]. Monoclonal antibodies were shown to lose almost 50% of neutralizing activity against B.1.1.7 carrying E484K. A combination of E484K with various NTD mutations (particularly deletions) might prove to be even more effective in immune evasion [
113], which is of the most significance in cases of both Beta variant and B1.1.7 with E484K.
The third globally recognized VOC, Gamma or P.1, is carrying 11 spike mutations. Five mutations are located within NTD (L18F, T20N, P26S, D138Y, R190S), three in RBD (K417T, E484K, N501Y), two in the C-terminal domain of S1 and near the furin cleavage site (D614G, H655Y), and one in S2 (T1027I) (
Figure 3). Convalescent and vaccinee sera show a significant loss of neutralizing activity against P.1, but the reduction is not as substantial as against B.1.315 [
114,
115,
116]. The loss of neutralizing activity of convalescent plasma against P.1 ranged from 6.5 to 13-fold and by sera of vaccinees from 2.2 to 2.8-fold [
114,
115], meaning that the neutralization of P.1 was not as severely compromised as that of B.1.351 and only slightly weakened compared to that of B.1.1.7. Not surprisingly, the neutralization activity of mAbs against P.1 is reduced much in the same manner as in B.1.351, since triple RBD mutations are mostly the same in both variants [
114].
The reason for the differences in neutralization of B.1.351 and P.1 by the immune serum presumably reflects the difference in the mutations introduced outside the RBD. The role of NTD-specific neutralizing antibodies is not nearly yet defined. It was thought that extensive N-linked glycan shielding of NTD is diminishing its antigenicity, but in vitro studies showed the significant neutralizing capacity of some NTD-specific antibodies [
52]. The fact that NTD is under selective pressure of human immune response is supported by the identification of NTD deletions in immunocompromised hosts with prolonged infections [
79]. It is possible that neutralization assays based on target cells over-expressing ACE2 receptors are responsible for underrating the role of NDT-specific antibodies. Since NTD changes are much more distinct among three major VOC, it seems likely that neutralization variation among them is rather due to differences in NTD than RBD.
In January 2021, the emergence of a novel variant in California carrying an L452R mutation in the RBD was reported [
77]. This variant (Epsilon) comprises two separate lineages B.1.427 and B.1.429, the first carrying two spike mutations (L452R, D614G) and the second carrying four (S13I, W152C, L452R, D614G). It is assumed that they emerged as early as May 2020 and they gained VOC status in the US due to significant increase in frequency from September 2020 to January 2021. In February 2021, they were identified in >50% of all sequenced cases in California and many other states [
117]. They were shown to display moderate resistance to neutralization by convalescent sera (4–6.7-fold) and sera of vaccine recipients (2–2.9-fold) [
48,
117]. The RBD mutation L452R, shared by these lineages, is not located in the part that directly interacts with ACE2, but it is speculated that it may cause structural changes in the region that promote the interaction between the spike protein and its ACE2 receptor. Thus, the infectivity of pseudoviruses carrying L452R was shown to be higher than of the D614G variant but slightly reduced compared to that of N501Y variants [
117]. The similar mechanism of RBD structural change due to L452R is offered in explanation of the reduced neutralization capacity of antibodies. This mutation, among several other RBD mutations, was selected by a panel of antibodies in vitro [
112].
The emerging variant B.1.617 comprises three distinct sublineages (B.1.617.1, B.1.617.2, B.1.617.3) with different mutational profiles [
70]. However, only sublineage B.1.617.2 or Delta is now internationally recognized as VOC. It is characterized by spike mutations T19R, G142D, Δ157-158, L452R, T478K, D614G, P681R, and D950N (
Figure 3). The other two sublineages have a similar mutational profile: B.1.617.1 is defined by the spike amino acid changes G142D, E154K, L452R, E484Q, D614G, P681R, and Q1071H, and B.1.617.3 is defined by T19R, L452R, E484Q, D614G, P681R, and D950N. The presence of RBD mutations L452R, E484Q, and D614G in the C-terminal domain of S1 may result in the higher transmissibility of these sublineages due to their known impact on ACE2 binding and conformational changes important for ACE2 binding. All three sublineages of B.1.617 display P681R adjacent to the furin cleavage site and have enhanced S cleavage by furin, which is hypothesized to be enhancing transmissibility and pathogenicity [
118]. Although the sublineage B.1.617.2 was initially considered to be as transmissible as B.1.1.7 [
119], further evidence from the UK, based on the likelihood that close contacts of a person infected with the Delta variant will themselves become infected—the “secondary attack rate”, suggest that this variant may be over 60% more transmissible than the Alpha variant [
120]. By recent report, more than 90% of new COVID-19 cases in the UK involve the Delta variant. The spread of the Delta variant is also registered in the US, where it now accounts for more than 6% of all infections (more than 18% of cases in some Western U.S. states) [
121].
The impact on the immune escape capacity of three sublineages of B.1.617 is expected, owing to RBD mutations L452R, T478K, and E484Q and their combination with NTD mutations and deletions, particularly in the case of B.1.617.2. A similar change at position 478 (T478I) was previously selected in vitro and shown to exhibit reduced neutralization by monoclonal antibodies and human convalescent sera [
112]. One of the first studies on B1.617.1 revealed that the neutralization capacity of convalescent sera and sera of recipients of inactivated killed vaccine was retained [
122]. Other studies have reported a moderate reduction in neutralization of B1.617.1 by the sera of convalescents and recipients of mRNA vaccines and resistance to monoclonal antibodies approved for COVID-19 treatment [
123,
124,
125]. The E484Q was found to have slightly milder impact but still corresponding to the effect of E484K, which is 10-fold reduction in the neutralization by sera of vaccine recipients. In addition, the combination of L452R and E484Q was not shown to have an additive effect; rather, the loss of sensitivity was similar to that observed with each mutation individually [
124].
Finally, the impact of emerging SARS-CoV-2 variants of concern on cellular immune response should also be addressed in future research. It has been suggested that the resolution of SARS-CoV-2 infection and COVID-19 is significantly dependent on CD4+ and CD8+ T-cell responses [
126], which also play a role in modulating disease severity [
45,
127]. In convalescent individuals, T-cell immunity is not restricted to spike-derived epitopes, and thus, it would be reasonable to assume that it would remain largely intact for new variants. However, in recipients of the majority of currently available vaccines, which offer S protein as the target immunogen, T-cell immunity is limited to spike epitopes. Therefore, it is of essence to determine whether new variant mutations in these epitopes impair T-cell responses in a similar way as escape from neutralizing antibodies. However, studies dealing with this problem are still scarce, mainly because measurements of T-cell immunity are more challenging for routine clinical practice than antibody detection assays. So far, the effect of variants B.1.1.7, B.1.351, P.1, and B.1.427/29 was found to be negligible on both CD4+ and CD8+ T-cell responses in the recipients of mRNA-based vaccines [
128,
129]. This was supported by the result of completely conserved epitopes for 93% of CD4+ and 97% of CD8+ T-cells in the variants. In addition, it was pointed out that HLA binding capacity is not affected in the majority of cases by a single mutation in epitopes. However, the repertoire of recognized epitopes is probably substantially different from one individual to the other due to HLA polymorphism, and thus, the negative impact of the mutations of specific variants on each single person could not be entirely dismissed [
128].